Abstract
Agenesis or congenital hypoplasia of skeletal muscles occurs infrequently but may occur with specific conditions such as Poland syndrome. The trapezius muscle can vary in the extent of its bony attachments or may have additional slips, however congenital absence or hypoplasia is extremely rare. There are only a few reports of partial or complete absence of the trapezius muscle. Two cases of bilateral absence of the trapezius were both in males and were accompanied by the absence of additional muscle in the pectoral girdle. Herein, we describe a case of a 56-year-old male cadaver with bilateral hypoplasia of the trapezius. The muscle was largely represented by atrophied muscle fibers with an abundance of fibrotic or fatty connective tissue. This subject had very minor hypoplasia of the left pectoralis major muscle, but the remaining muscles of the pectoral girdle were normal. The spinal accessory nerve terminated in the sternocleidomastoid muscle on both sides, failing to reach the trapezius. We interpret these findings to be consistent with a minor variant of Poland syndrome.
The trapezius muscle is derived from neural crest cells and paraxial mesenchyme in the head and typically exists as a broad, flat triangular muscle situated superficially along the posterior aspect of the neck and thorax [1]. Both trapezius muscles attach along the posterior aspect of the occipital bone, ligamentum nuchae, and spinous processes extending from C7 to T12. These medial attachments are through tendinous insertions, however from C6 to T3 these become aponeurotic. The trapezius is described as existing in three portions based on attachments. The superior fibers descend to the lateral aspect of the clavicle, the middle fibers run transversely to the acromion and superior border of the spine of the scapula and the inferior fibers attach along the inferior border of the scapular spine. Extrafusal fibers of the trapezius are innervated by lower motor neurons in the lateral aspect of the anterior horn of the C1-C4 spinal cord (i.e., the accessory nucleus) and lower motor neurons in the ventral horn of C3-4 spinal cord and intrafusal fibers are innervated by neurons in the posterior root ganglia of C3-4 [2, 3].
The trapezius is not frequently associated with significant anatomical variation. The most common variations involve the extent of its attachments. Specifically, the muscle may fail to extend superiorly to the occipital bone or it may not extend below the spinous processes of T8. Hypoplasia or agenesis of the trapezius is much more rare. The inferior portion of the muscle has been reported as hypoplastic or even absent and replaced with aponeurotic fibers [4]. Complete unilateral absence of the trapezius has been reported [5, 6]. Allouh and coworkers [5] reported on a cadaveric specimen with complete absence of muscle and even connective tissue elements in the region of the left trapezius, although the right trapezius was normal (Figs 1, 2). Chawla and colleagues [6] reported on a male subject with congenital torticollis and absence of the right sternocleidomastoid and trapezius. There are two reports of individuals with bilateral absence of the trapezius [7, 8], both of which were missing additional muscles in their pectoral girdle. Specifically, Gross-Kieselstein and Shalev [8] reported on two siblings with bilateral absence of the trapezius with hypoplasia of pectoralis major, supraspinatus and serratus anterior muscles. Horan and Bonafede [7] reported on a subject with bilateral absence of the trapezius muscle and sternal head of pectoralis major. These three subjects were all male, raising the distinct possibility of a congenital muscular disorder [8]. Nonetheless, congenital absence of the trapezius is a rare occurrence, and when it occurs it is likely part of more extensive muscle agenesis or hypoplasia. The observations within this case study appear to not have been reported previously. Specifically, no other published literature with comparable findings were identified during our research. Therefore, a careful description of these findings are important and will serve as foundation for future research on the rare and variable effects associated with Poland syndrome.
Routine dissection of a 56-year-old male cadaver who died of natural causes revealed bilateral hypoplasia of the trapezius muscle (Fig. 1A). Much of the extent of the hypoplastic trapezius was occupied by fibrous and occasionally fatty connective tissue. The inferior aspects of the hypoplastic trapezius were largely aponeurotic while the superior aspect was infiltrated with adipose, especially on the left (see below; Fig. 2). There were some regions that contained obvious skeletal muscle fibers (Fig. 1A, black arrows). Specifically, on the right side only the superior most aspect and a small island within the inferior part contained skeletal muscle fibers (Fig. 1A, black arrows). Besides the paucity of skeletal muscle fibers, the hypoplastic trapezius in this subject only extended as far superiorly as the ligamentum nuchae at the level of C3 and only as far inferior as T9 (Fig. 1A). On the left, the hypoplastic trapezius had no grossly discernable skeletal muscle fibers; accordingly, no branches from the spinal accessory nerve (CN XI) or the cervical plexus could be traced into it; vascular support on the deep surface was scant. On the right, the hypoplastic trapezius only received innervation from the cervical plexus (Fig. 1B, yellow wire). The right CN XI was traced into the sternocleidomastoid muscle but terminated within the muscle belly (Fig. 1B, black wire and arrowhead); no branches from CN XI were found to extend beyond the sternocleidomastoid muscle. There was no evidence of surgery, trauma or radiation therapy that might have impacted CN XI in the posterior triangle of the neck. Two tissue samples, approximately 1 cubic centimeter each, were processed for histological study (see red boxes on Figs. 1A, 2).
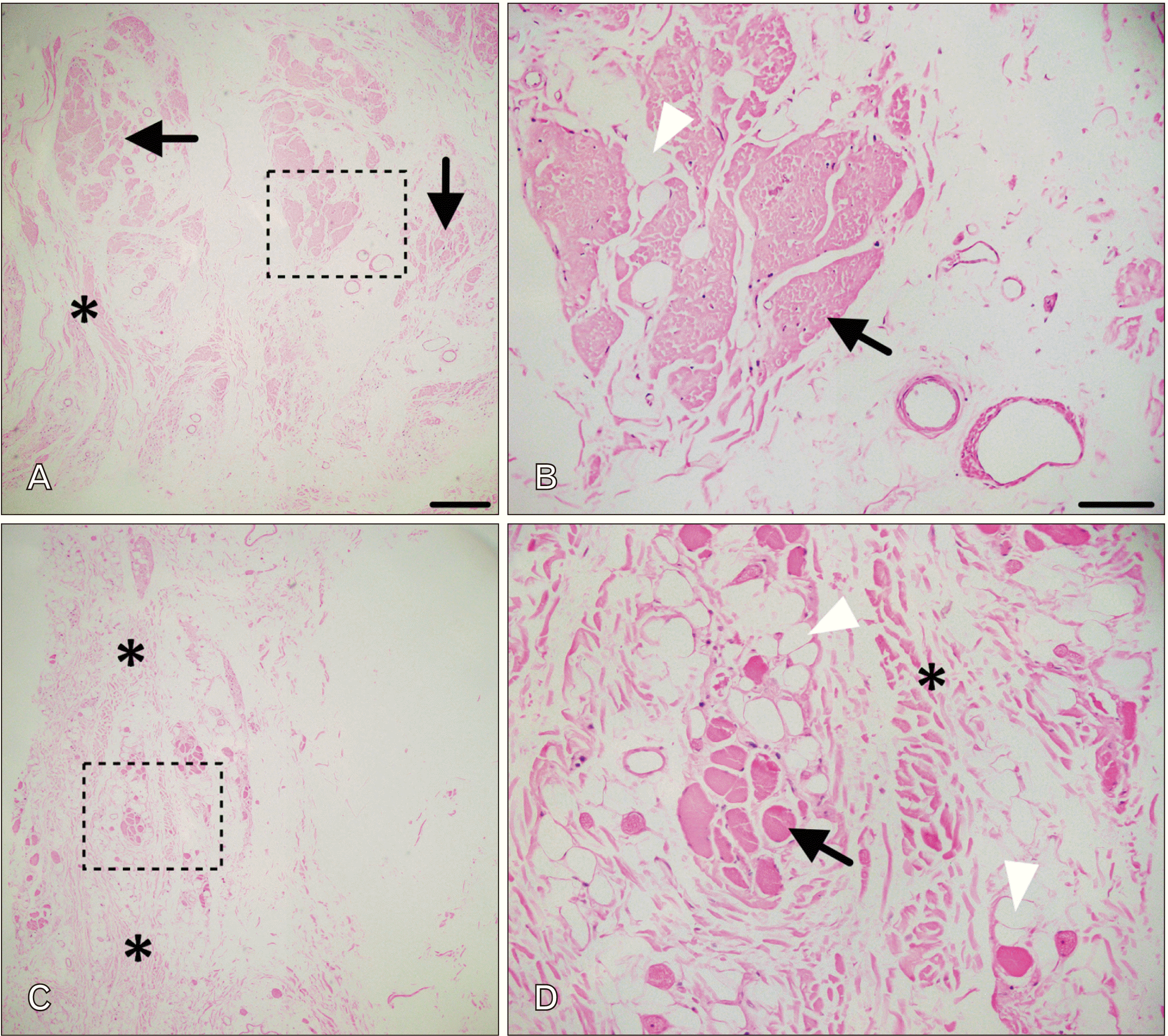
Examination of H&E-stained sections from the regions indicated above were consistent with hypoplasia. Microscopic examination of 5 µm thick sections from the left trapezius (Fig. 2A, B) revealed pale, poorly organized and atrophied muscle fibers (arrows in Fig. 2A). These fibers were separated by dense, but disorganized bundles of collagen fibers (asterisks). A higher magnification view of the region indicated by the black box is shown in B. The black arrow indicates an atrophied fascicle infiltrated with adipose (white arrowhead); no clear fiber arrangement is evident. Microscopic examination of 5 µm thick sections from the right trapezius revealed few isolated bundles of skeletal muscle (Fig. C, D). These islands of muscle were separated by dense connective tissue (asterisks). The region indicated by the black box is shown at higher magnification in D. A small collection of muscle fibers is indicated by the black arrow. The muscle fibers that were identified varied markedly in size and shape and were surrounded by collagen fibers (asterisk) and infiltrated with adipose (white arrowhead).
Since hypoplasia of the trapezius appears to be commonly associated with dysmorphology or agenesis of other muscles in the pectoral girdle, we completed dissection of these regions bilaterally. Muscles of the pectoral girdle were grossly intact with only a single, very minor alteration. Both sternocleidomastoid muscles appeared normal bilaterally (Fig. 1C). The right pectoralis major muscle was normal but the left pectoralis major was missing the most inferior fibers (Fig. 1C, black arrowheads). Both pectoralis minor muscles were symmetric and of normal size. Finally, both levator scapulae muscles were present, symmetric and innervated on their deep surface by the dorsal scapular nerve (Fig. 1B).
The subject of this case study had severe bilateral hypoplasia of the trapezius. The left trapezius consisted of either aponeurotic fibers or dense regular connective tissue with fatty infiltration. The upper portion of the right trapezius included skeletal muscle as well as a small island in the inferior portion; the rest was aponeurotic or dense regular connective tissue. This subject had no other gross muscle abnormalities in the pectoral girdle, but there was very mild hypoplasia of the left pectoralis major muscle. In hypoplasia as severe as noted in this subject, there was very likely compensatory changes in shoulder girdle muscles with functions similar to the trapezius to allow for normal strength and range of motion. With bilateral hypoplasia of the trapezius, we would predict adaptive changes in the levator scapulae, rhomboid major and minor, serratus anterior and latissimus dorsi muscles. There was no evidence of nerve trauma or injury in the posterior triangle of the neck that would have impacted CN XI. We believe these findings are consistent with developmental etiologies and may represent a rare variation of Poland syndrome.
Bilateral hypoplasia or agenesis of the trapezius is an extremely rare occurrence and the only reports of this in the literature are associated with Poland syndrome [9, 10]. Poland syndrome is a rare congenital anomaly with an incidence of 1 in 30,000 [11]. This condition is characterized by congenital defect of the pectoralis major muscle and severity ranges from partial to complete absence of the pectoralis major and may include various other muscles of the pectoral girdle [10]. Poland syndrome is unilateral in 60%–70% of cases [12] with the right side being most commonly affected. Interestingly, the trapezius muscle is affected in only 7% of subjects with Poland syndrome [10]. Based on the aforementioned estimates, we believe bilateral hypoplasia of the trapezius would occur with an approximate incidence of 1 in 1.5 million subjects. The etiology of Poland syndrome is unclear. There is very likely a genetic component as incidence is higher in siblings [8]. However, disruption of arterial support arising from the subclavian artery is the most accepted etiology [13]. Indeed, we found very small supporting vasculature on the deep surface of the left hypoplastic trapezius, but these vessels were larger on the right side. Consistent with the absence of muscle fibers, we found no nerves from CN XI or cervical plexus on the deep surface of the hypoplastic left trapezius. On the right, there were only branches from the cervical plexus. As such, the small amount of skeletal muscle on the right was most likely innervated from motor axons from the cervical plexus [2, 3]. We were unable to find any reports of CN XI failing to innervate the trapezius, but CN XI has been reported to terminate in the sternocleidomastoid [14].
Regardless of the etiology, congenital absence of the trapezius will undoubtedly result in impaired shoulder range of motion and abnormal posturing of the pectoral girdle. Such displacement may result in compression of the axillary sheath, resulting in impaired venous and lymphatic return and signs/symptoms of compressive neuropathy. Although extremely rare, it is important for clinicians to recognize variants of Poland syndrome, the possible involvement of the trapezius in congenital syndromes and the impact absent muscles can have on structure and function of the upper extremity.
In conclusion, this case study describes a subject with severe bilateral hypoplasia of the trapezius confirmed by histological examination. Mild involvement of the left pectoralis major suggests this subject may have an extremely rare variant of Poland syndrome.
Acknowledgements
The authors are grateful to those who donated their bodies to medical education and anatomical research. Results from such anatomical research provides an important avenue to extend our understanding of human structure and function and improve patient care.
Notes
References
1. Standring S. 2016. Gray's anatomy: the anatomical basis of clinical practice. 41st ed. Elsevier;Philadelphia:
2. McKenzie J. 1955; The morphology of the sternomastoid and trapezius muscles. J Anat. 89:526–31. PMID: 13278302. PMCID: PMC1244744.
3. Tubbs RS, Shoja MM, Loukas M, Lancaster J, Mortazavi MM, Hattab EM, Cohen-Gadol AA. 2011; Study of the cervical plexus innervation of the trapezius muscle. J Neurosurg Spine. 14:626–9. DOI: 10.3171/2011.1.SPINE10717. PMID: 21388290.

4. Garbelotti Júnior SA, Rodrigues CF, Sgrott EA, Prates JC. 2001; Unilateral absence of the thoracic part of the trapezius muscle. Surg Radiol Anat. 23:131–3. DOI: 10.1007/s00276-001-0131-x. PMID: 11462862.

5. Allouh M, Mohamed A, Mhanni A. 2004; Complete unilateral absence of trapezius muscle. McGill J Med. 8:31–3. DOI: 10.26443/mjm.v8i1.375. PMID: d7671205ada44a97ad55cd2f8bcff149.

6. Chawla S, Tandon A, Meena G. 2021; Unilateral absence of sternocleidomastoid and ipsilateral trapezius presenting as congenital torticollis: a case of a rare entity. Cureus. 13:e17222. DOI: 10.7759/cureus.17222. PMID: 34540449. PMCID: PMC8442799.

7. Horan FT, Bonafede RP. 1977; Bilateral absence of the trapezius and sternal head of the pectoralis major muscles. A case report. J Bone Joint Surg Am. 59:133. DOI: 10.2106/00004623-197759010-00029. PMID: 833166.

8. Gross-Kieselstein E, Shalev RS. 1987; Familial absence of the trapezius muscle with associated shoulder girdle abnormalities. Clin Genet. 32:145–7. DOI: 10.1111/j.1399-0004.1987.tb03344.x. PMID: 3621659.

9. Blanco FC, Elliott ST, Sandler AD. 2011; Management of congenital chest wall deformities. Semin Plast Surg. 25:107–16. DOI: 10.1055/s-0031-1275177. PMID: 22294949. PMCID: PMC3140238.

10. Yiyit N, Işıtmangil T, Öksüz S. 2015; Clinical analysis of 113 patients with Poland syndrome. Ann Thorac Surg. 99:999–1004. DOI: 10.1016/j.athoracsur.2014.10.036. PMID: 25633462.

11. Freire-Maia N, Chautard EA, Opitz JM, Freire-Maia A, Quelce-Salgado A. 1973; The Poland syndrome-clinical and genealogical data, dermatoglyphic analysis, and incidence. Hum Hered. 23:97–104. DOI: 10.1159/000152560. PMID: 4356989.

12. Shamberger RC, Welch KJ, Upton J 3rd. 1989; Surgical treatment of thoracic deformity in Poland's syndrome. J Pediatr Surg. 24:760–5. discussion 766DOI: 10.1016/S0022-3468(89)80532-9. PMID: 2549232.

13. Bavinck JN, Weaver DD. 1986; Subclavian artery supply disruption sequence: hypothesis of a vascular etiology for Poland, Klippel-Feil, and Möbius anomalies. Am J Med Genet. 23:903–18. DOI: 10.1002/ajmg.1320230405. PMID: 3008556.

14. Quain J, Schaefer EA, Thane GD. 1897. Quain's Elements of anatomy. 10th ed. Longmans, Green;London:
Fig. 1
Dissection of T muscles. Shown in (A) is a superficial dissection of the hypoplastic T (outlined with black dashed line). The only muscle fibers present are indicated by black arrows. The regions indicated by the red boxes were removed for histological study (Fig. 2). The region indicated by the green box was a defect revealing the underlying rhomboid muscle. The blue box indicates the region of a deeper dissection shown in B. (B) shows the underside of the S and T. The spinal accessory nerve (black wire/arrowhead) terminates within the S. The greater occipital nerve is indicated by the red wire and red arrowhead. Branches from the cervical plexus (yellow wire/yellow arrowheads) were found along the deep surface of the hypoplastic T. (C) shows the anterior neck and shoulder region from the subject. The S muscles were symmetric and appeared of normal bulk. The right PM muscle appeared normal, the left PM appeared to be missing the inferiormost fibers (arrowheads). D: deltoid, ES: erector spinae, L: latissimus dorsi, LS: levator scapulae, PM: pectoralis major, R: rhomboid major, S: sternocleidomastoid, SH: sternohyoid, Sp: splenius, SS: semispinalis, T: trapezius.
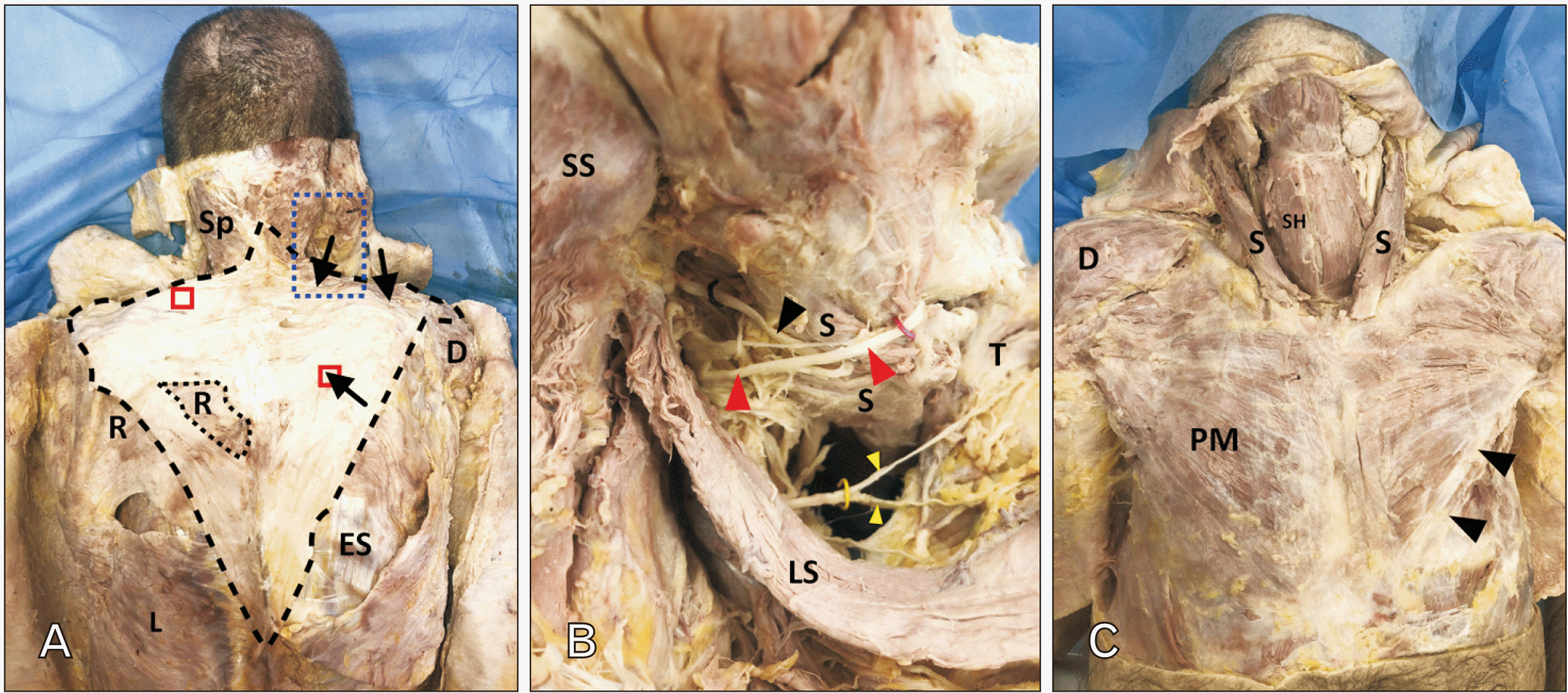
Fig. 2
Histological examination. Shown in (A, B) are H&E-stained sections from the left trapezius. There were few characteristic skeletal muscle fascicles present (black arrows), but there was an abundance of collagen fibers (asterisk). The region indicated by the box is shown at higher magnification in B. The muscles fibers were pale, irregular and indistinct (black arrow), There was infiltration with adipose (white arrow). Shown in (C, D) are H&E-stained sections from the right trapezius. There were very few muscle fibers, although when present there had characteristic morphology. There was an abundance of connective tissue fibers (asterisks). The region in the box is shown at higher magnification in (D). Muscle fibers (black arrow) were surrounded by adipose (white arrowheads) and collagen. The scale bar in A is 400 microns and corresponds to C also. The scale bar in B is equal to 100 microns and corresponds to D also.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download