Introduction
Materials and Methods
Animals
Experimental substances
Experimental design
Evaluation methods
Pancreatic weight/rat weight ratio analysis
Biochemical analysis
Histological and immunohistochemical assessments
Immunohistochemical study
Morphometrical studies
Statistical analysis
Results
Pancreatic weight (PW)/Rat weight (RW) ratio results
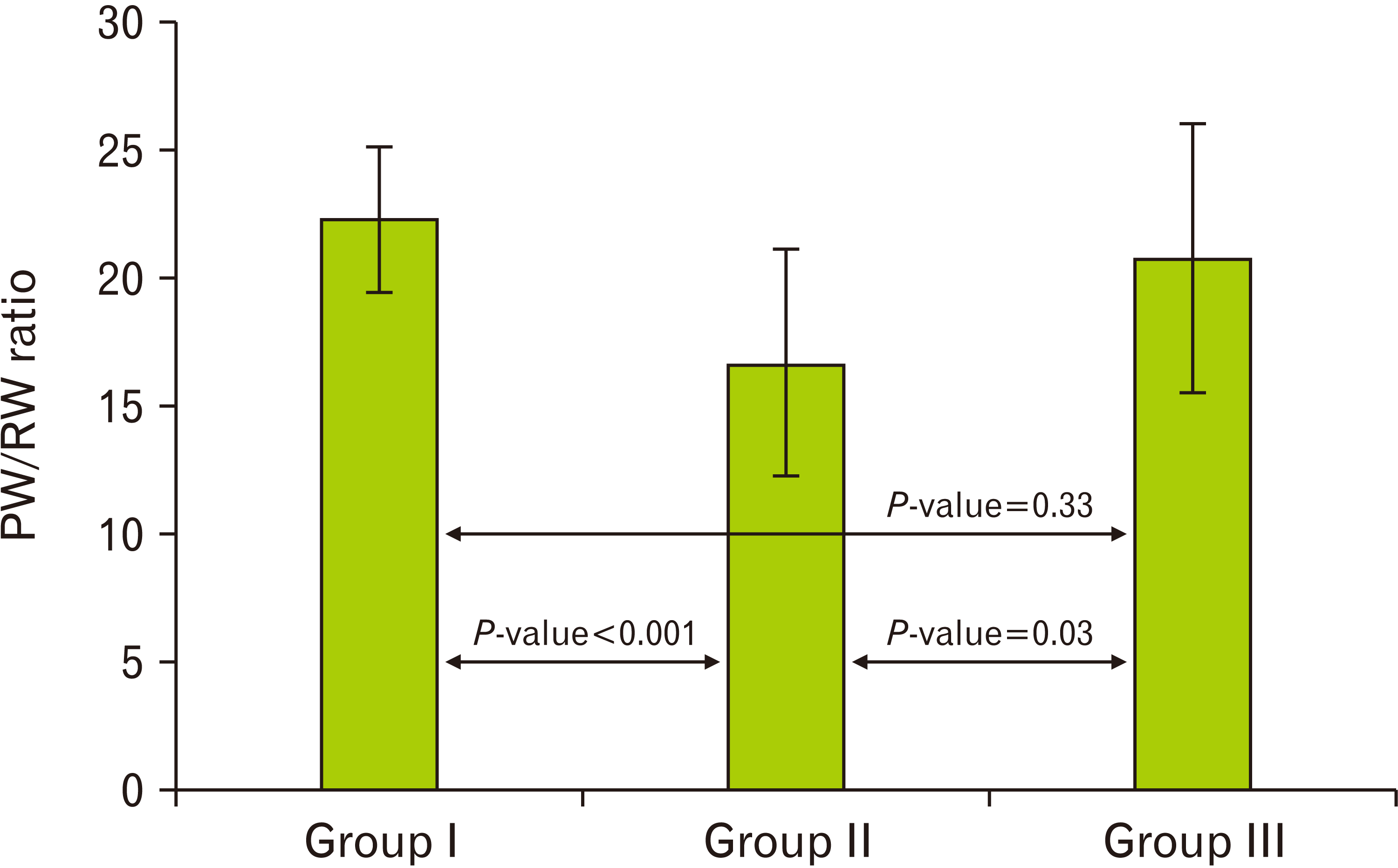 | Fig. 1A histogram demonstrating a highly significant decrease (P<0.001) in the PW/BW ratio in the cipralex group compared to the control group, while the cipralex plus SIL group, illustrating a significant increase compared to the cipralex group, with no significant difference between control and cipralex plus SIL groups. PW, pancreatic weight; RW, rat weight; SIL, silymarin. |
Table 1
| Weight parameter |
Group I (control) n=15 |
Group II (cipralex) n=15 |
Group III (cipralex plus SIL) n=15 |
t-test | P-value |
|---|---|---|---|---|---|
| PW | 5.06 | <0.001a | |||
| 4.17±0.64 | 2.72±0.65 | 3.43±0.87 | 1.95 | 0.06b | |
| 3.1–5.0 | 1.6–3.6 | 2.2–4.6 | 2.78 | 0.01c | |
| RW | 4.83 | <0.001a | |||
| 186.93±11.53 | 167.07±10.98 | 178.47±13.24 | 1.87 | 0.07b | |
| 165–210 | 150–185 | 155–194 | 2.57 | 0.02c | |
| PW/RW ratio | 4.13 | <0.001a | |||
| 22.29±2.83 | 16.66±4.46 | 20.76±5.28 | 0.98 | 0.33b | |
| 18.24–26.74 | 8.65–23.78 | 11.58–29.68 | 2.30 | 0.03c | |
Biochemical results
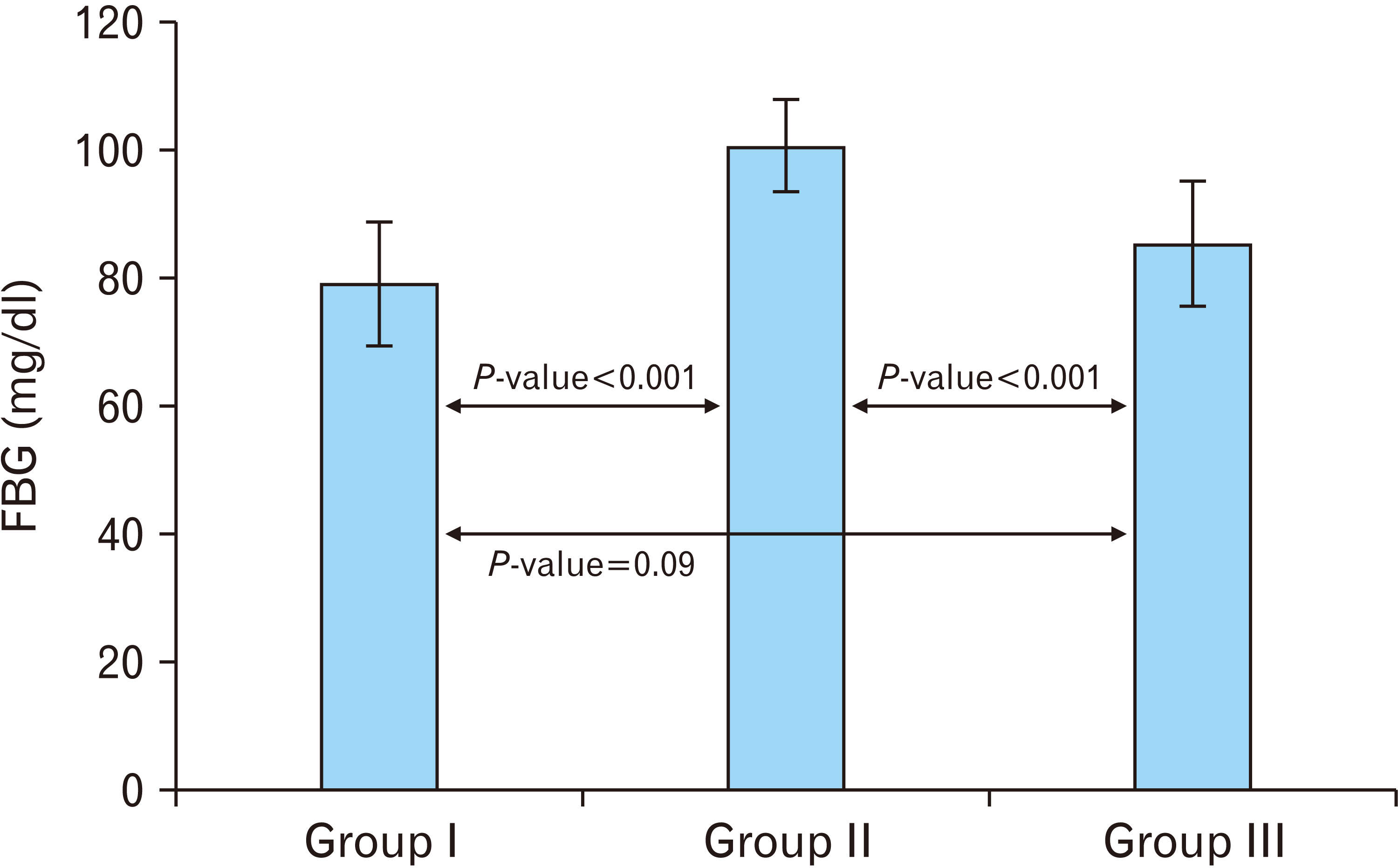 | Fig. 2A histogram illustrating a highly significant increase (P<0.001) in FBG in the cipralex group compared to the control group, while the cipralex plus SIL group, illustrating a high significant decrease compared to the cipralex group, with no significance between control and cipralex plus SIL groups. SIL, silymarin; FBG, fasting blood glucose. |
Table 2
| Biochemical analysis parameter |
Group I (control) n=15 |
Group II (cipralex) n=15 |
Group III (cipralex plus SIL) n=15 |
t-test | P-value |
|---|---|---|---|---|---|
| Fasting blood glucose (mg/dl) | 6.80 | <0.001a | |||
| 79.13±9.62 | 100.53±7.21 | 85.33±9.66 | 1.74 | 0.09b | |
| 60–95 | 88–111 | 70–100 | 4.88 | <0.001c | |
| Insulin level (U/l) | 4.18 | <0.001a | |||
| 2.39±0.29 | 1.93±0.32 | 2.22±0.34 | 1.51 | 0.14b | |
| 1.9–2.8 | 1.5–2.5 | 1.3–2.6 | 2.42 | 0.02c | |
| Serum amylase level (U/l) | 12.19 | <0.001a | |||
| 763.73±37.41 | 586.07±42.26 | 757.47±27.83 | 0.52 | 0.61b | |
| 666–800 | 499–678 | 708–808 | 13.12 | <0.001c |
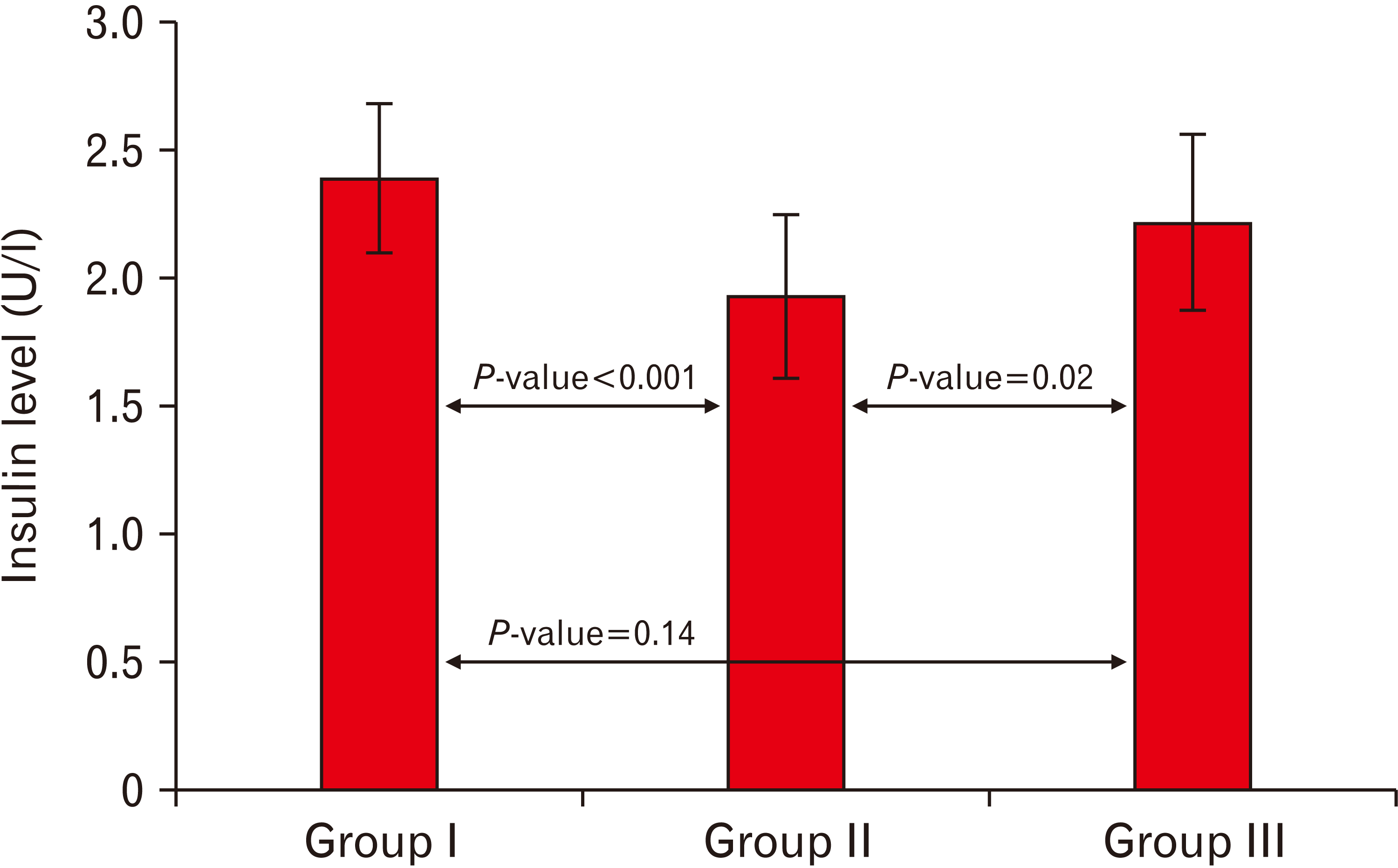 | Fig. 3A histogram showing a highly significant decrease (P<0.001) in the insulin level in the cipralex group compared to the control group, while the cipralex plus SIL group, illustrating a significant increase compared to the cipralex group, with no significant difference between control and cipralex plus SIL groups. SIL, silymarin. |
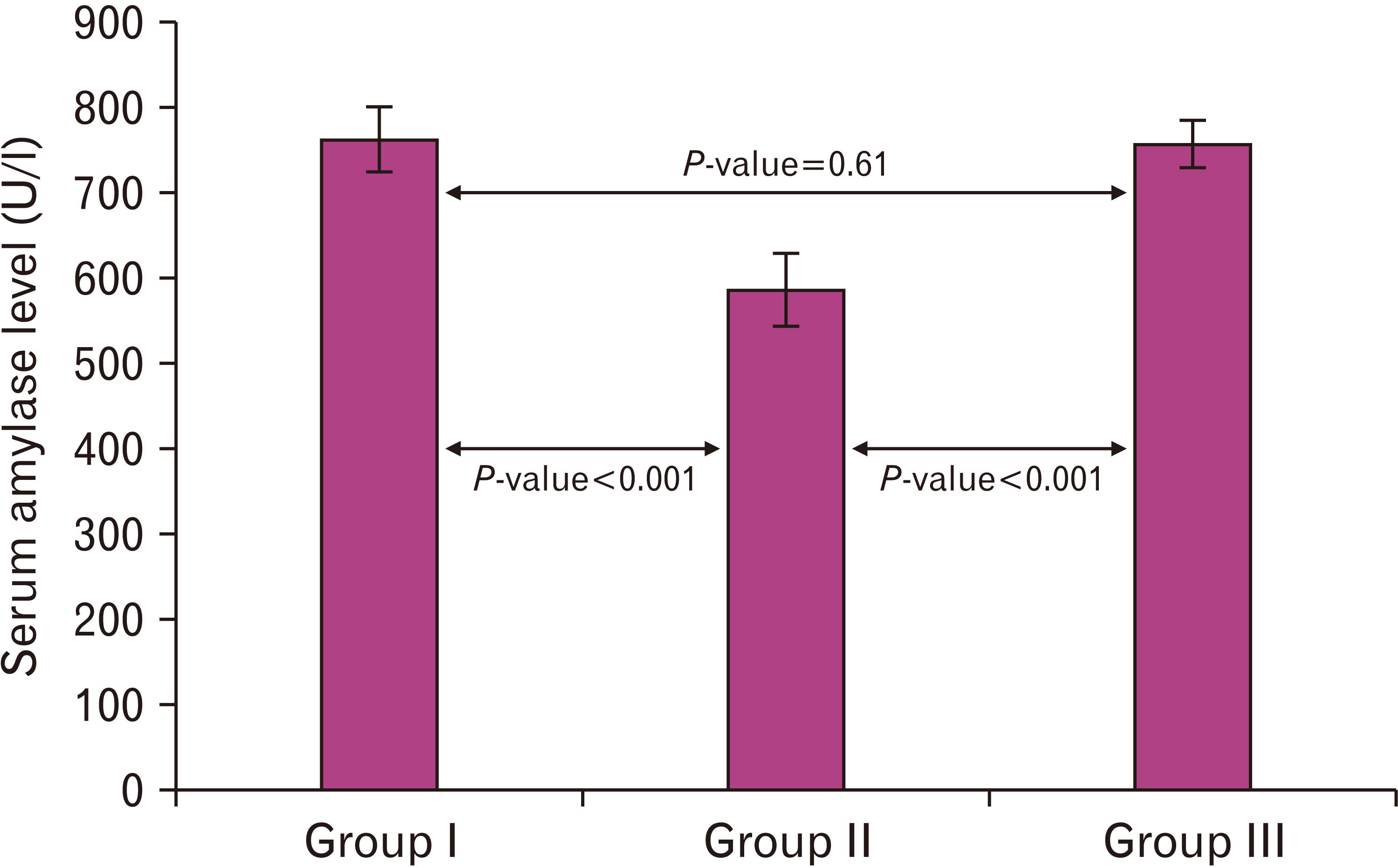 | Fig. 4A histogram demonstrating a highly significant decrease (P<0.001) in the amylase level in the cipralex group compared to the control group, while the cipralex plus SIL group, illustrating a high significant increase compared to the cipralex group, with no significance between control and cipralex plus SIL groups. SIL, silymarin. |
Light microscopic examination
Hematoxylin and eosin stain results
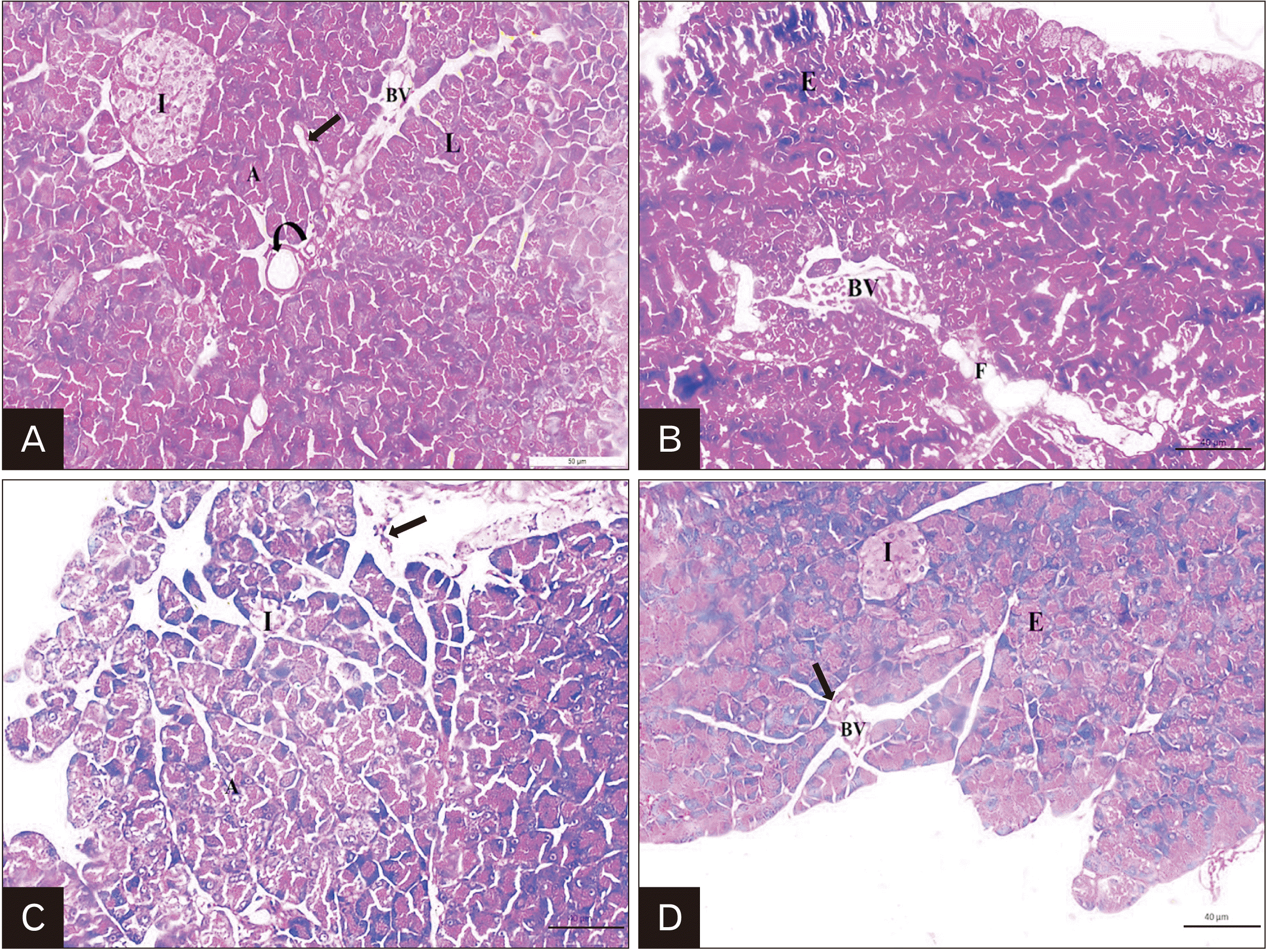 | Fig. 5Representative H&E staining of rat pancreas; (A) Control group: demonstrating that the pancreas is formed of lobules (L) of different sizes and shapes separated by thin connective tissue septa (arrow). Interlobular duct (curved arrow) and blood vessel (BV) are observed. Each lobule is formed of highly packed darkly stained acini (A) (the exocrine portion of the pancreas). The islet of Langerhans (I) (the endocrine portion of the pancreas) is appeared as large defined pale stained area scattered between the darkly stained pancreatic acini. (B) Cipralex group: showing destruction of the normal pancreatic architecture of the exocrine part (E), congested interlobular BV and distorted fat cells (F) are observed. (C) Cipralex group also illustrating shrunken and vaguely identified pancreatic islet (I), distorted acini (A). Inflammatory cell infiltration (arrow) are also observed. (D) Cipralex plus SIL group: showing restoration of the normal architecture of the E and endocrine part (I). Interlobular BV and duct (arrow) appear normal in between the cells (×200, scale bar=40 μm). |
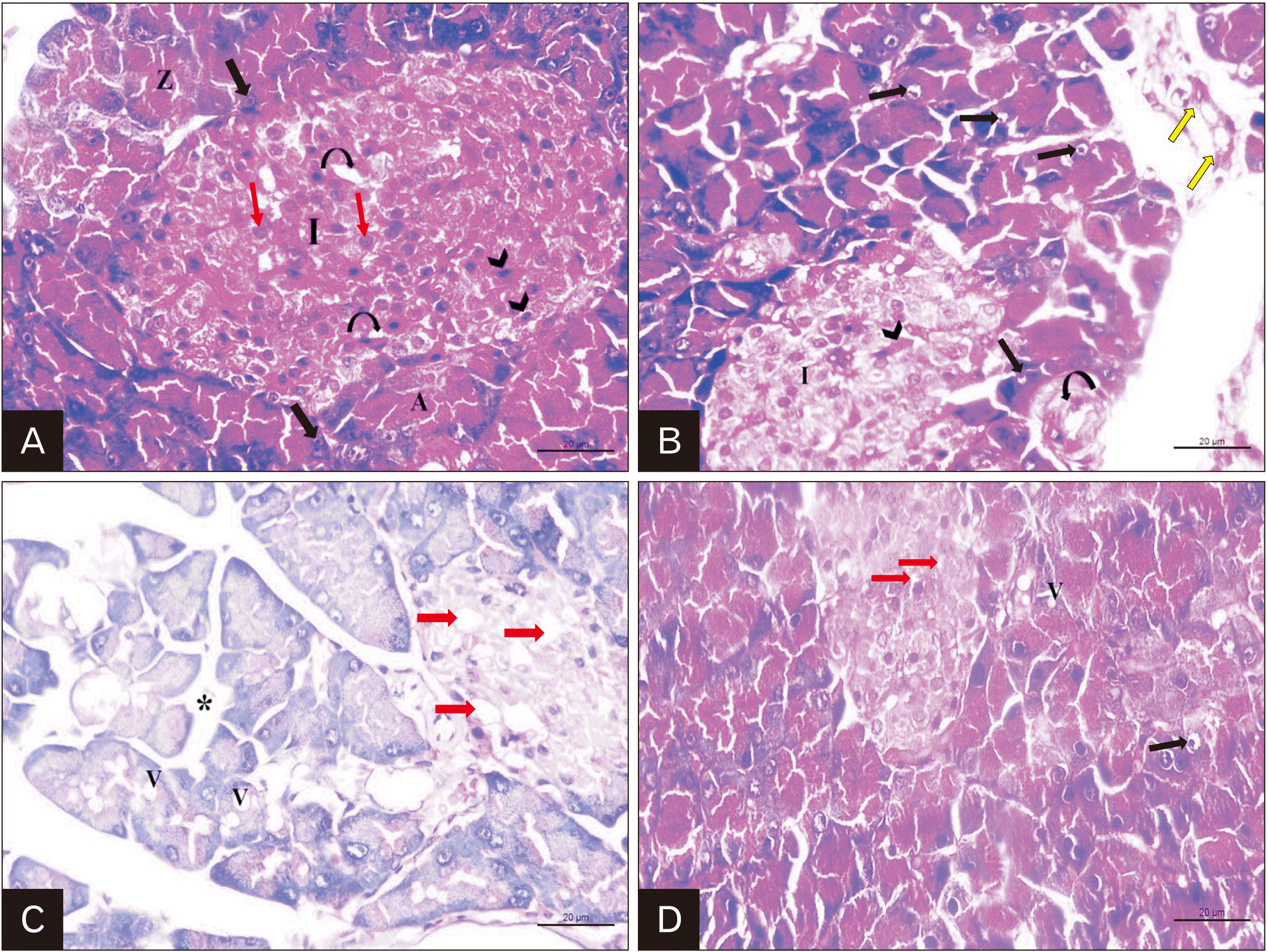 | Fig. 6Representative H&E staining of rat pancreas; (A) Control group: illustrating that the pancreatic acini (A) have basal basophilic cytoplasm containing rounded vesicular nuclei (black arrow) and apical acidophilic zymogen granules (Z). The islet of Langerhans (I) is composed of cords of secretory cells separated by blood capillaries (curved arrow). Two types of cells are recognized; central beta cells with rounded and lighter nuclei (red arrow) and peripheral alpha cells with oval darkly stained nuclei (arrow head). (B) Cipralex group: showing that many acinar cells have darkly stained pyknotic nuclei (black arrow). Distorted shape of islet cells with apparent decrease in the number (I) and congested blood capillaries (arrow head) are noticed. Thickening in the wall of pancreatic duct (curved arrow) and thickening of the interlobular septa are observed (yellow arrow). (C) Cipralex group: also demonstrating that most of pancreatic acinar cells have cytoplasmic vacuolations (V) and many β-cells of islet of Langerhans are lost and marked vacuolations (red arrow) are seen. Empty spaces (*) are found between pancreatic acini. (D) Cipralex plus silymarin group: showing few acinar cells with pyknotic nuclei (black arrow) and some cells have mild vacuolated cytoplasm (v). Few β-cells appear vacuolated (red arrow) (×400, scale bar =20 μm). |
Masson’s trichrome stain results
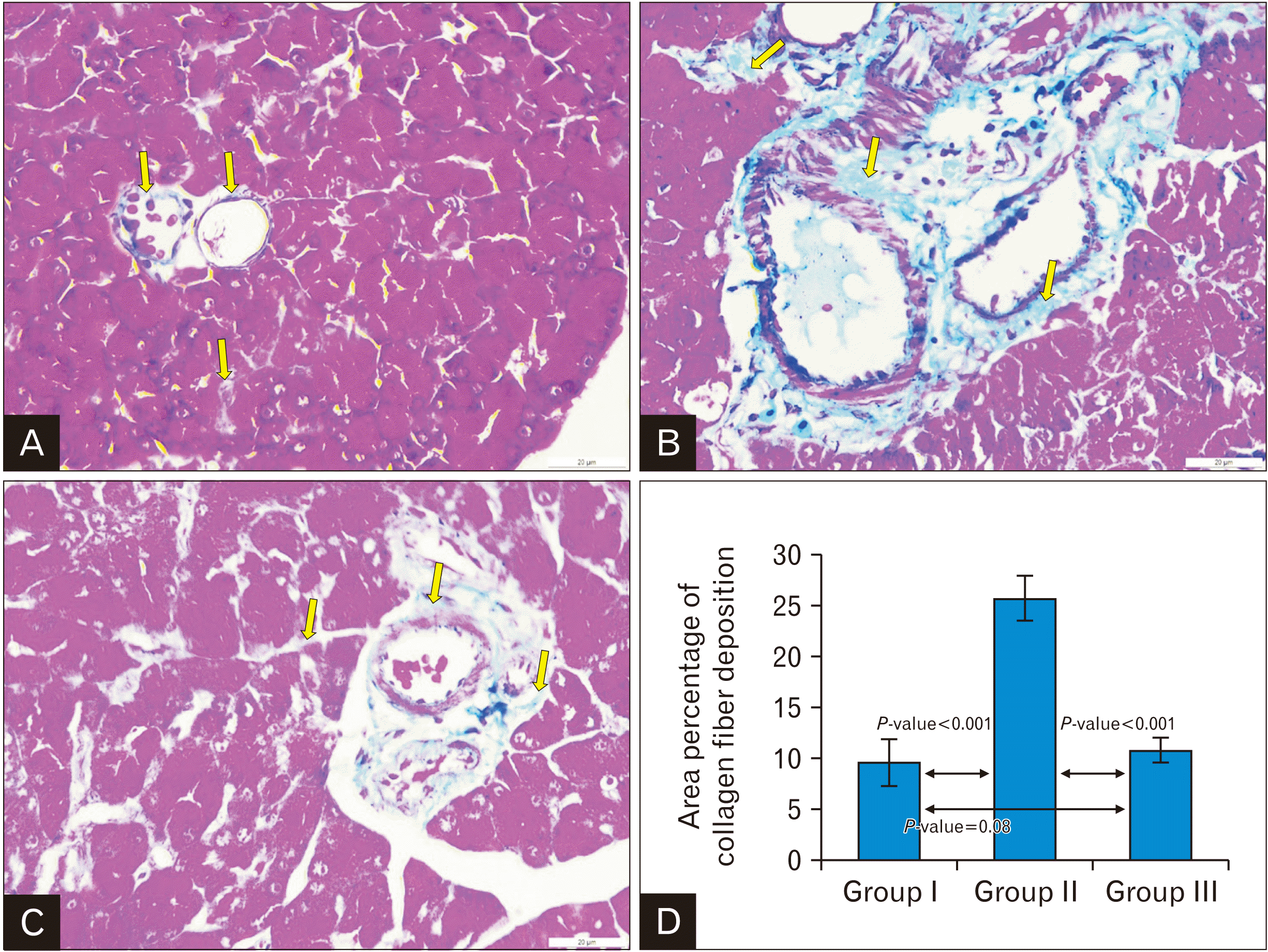 | Fig. 7Representative Masson’s Trichrome stain of rat pancreas; (A) Control group: showing delicate collagen fibers around the pancreatic acini, pancreatic ducts and blood vessels (yellow arrow). (B) Cipralex group: showing dense collagen fiber deposition (yellow arrow). (C) Cipralex plus SIL group: showing moderate collagen fiber deposition (yellow arrow) as compared with cipralex group (yellow arrow) (×400, scale bar=20 μm). (D) A histogram demonstrating a highly significant increase in the mean area percentage of collagen fibers in cipralex group compared to control group. While cipralex plus SIL group showing a highly significant decrease compared to cipralex group, with no significant difference between control and cipralex plus SIL groups. SIL, silymarin. |
Immunohistochemical findings
 | Fig. 8Representative iNOS immunostaining of rat pancreas; (A) Control group: illustrating negative immunoreactivity in pancreatic acini and islet cells. (B) Cipralex group: illustrating strong cytoplasmic immunoreactivity in most pancreatic acini and islet cells. (C) Cipralex plus SIL group: illustrating mild cytoplasmic immunoreactivity in pancreatic acini and islet cells as compared with cipralex group (×400, scale bar=20 μm). (D) A histogram illustrating a highly significant increase in iNOS immune expression in cipralex group compared to control group. While cipralex plus SIL group showing a highly significant decrease compared to cipralex group, with no significance between control and cipralex plus SIL groups. iNOS, inducible nitric oxide synthase; SIL, silymarin. |
 | Fig. 9Representative TNF-α immunostaining of rat pancreas; (A) Control group: revealing negative immunoreactivity in pancreatic acini and islet cells. (B) Cipralex group: showing strong immunoreactivity in most of pancreatic acini and islet cells. (C) Cipralex plus SIL group: showing mild immunoreactivity in pancreatic acini and islet cells as compared with cipralex group (×400, scale bar=20 μm). (D) A histogram showing a highly significant increase in TNF-α immune expression in cipralex group compared to control group. While cipralex plus SIL group showing a highly significant decrease compared to cipralex group, with no significant difference between control and cipralex plus SIL groups. TNF-α, tumour necrosis factor-alpha; SIL, silymarin. |
 | Fig. 10Representative caspase-3 immunostaining of rat pancreas; (A) Control group: demonstrating negative immunoreactivity in pancreatic acini and islet cells. (B) Cipralex group: demonstrating strong cytoplasmic and nuclear immunoreactivity for caspase-3 in most pancreatic acini and islet cells. (C) Cipralex plus SIL group: demonstrating moderate immunoreactivity in pancreatic acini and islet cells as compared with cipralex group (×400, scale bar=20 μm). (D) A histogram illustrating a highly significant increase in caspase-3 immune expression in cipralex group compared to control group. While cipralex plus SIL group demonstrating a highly significant decrease compared to cipralex group, with no significance between control and cipralex plus SIL groups. SIL, silymarin. |
 | Fig. 11Representative PCNA immunostaining of rat pancreas; (A) Control group: illustrating strong nuclear immunoreactivity pancreatic acini and islet cells. (B) Cipralex group: showing mild immunoreactivity for PCNA in most of pancreatic acini and islet cells. (C) Cipralex plus SIL group: showing moderate immunoreactivity pancreatic acini and islet cells as compared with cipralex group (×400, scale bar=20 μm). (D) A histogram exhibiting a highly significant decrease in PCNA immune expression in cipralex group compared to control group. While cipralex plus SIL group showing a highly significant increase compared to cipralex group, with no significant difference between control and cipralex plus SIL groups. PCNA, proliferating cell nuclear antigen; SIL, silymarin. |
 | Fig. 12Representative insulin immunostaining of rat pancreas; (A) Control group: revealing strong cytoplasmic immunoreactivity for insulin in most of the cells of the pancreatic islet. (B) Cipralex group: showing mild immunoreactivity for insulin in a small number of islets cells. (C) Cipralex plus SIL group: showing moderate cytoplasmic immunoreactivity for insulin in most of the pancreatic islet as compared with cipralex group (×400, scale bar=20 μm). (D) A histogram exhibiting a highly significant decrease in insulin positive β-cells immune expression in cipralex group compared to control group. While cipralex plus SIL group showing a significant increase compared to cipralex group, with no significant difference between control and cipralex plus SIL groups. SIL, silymarin. |
Morphometrical and Statistical analysis results
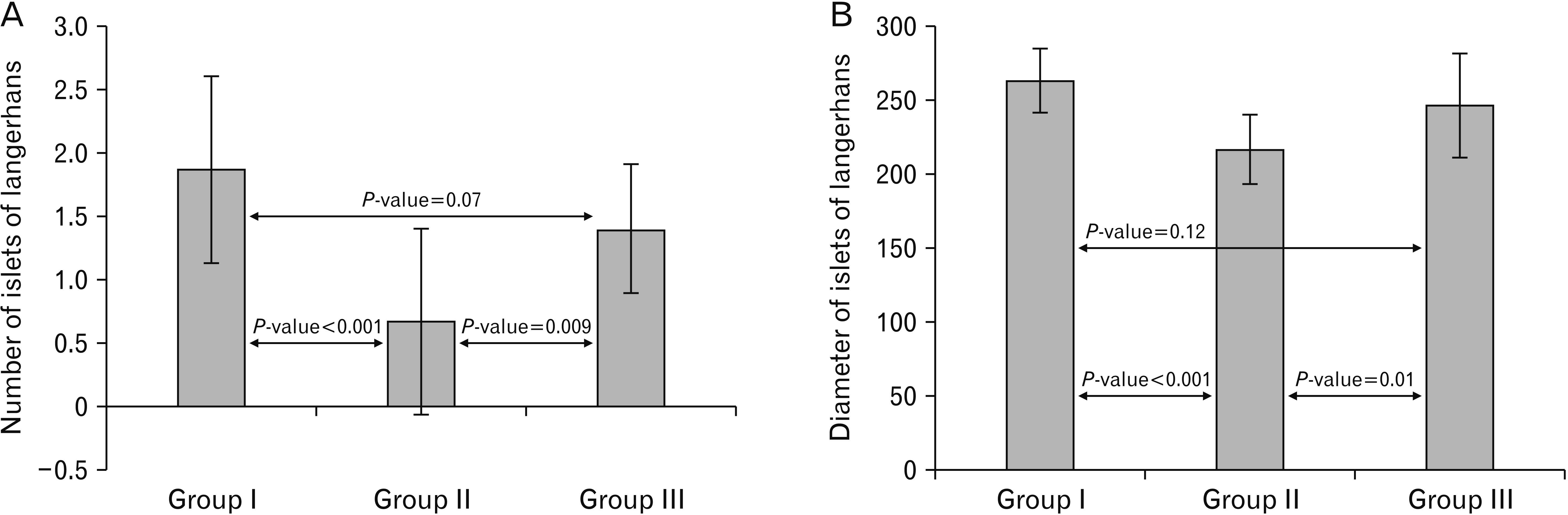 | Fig. 13A histogram showing a highly significant decrease (P<0.001) in the number and diameter of islets of Langerhans in the cipralex group compared to the control group, while the cipralex plus SIL group, illustrating a significant increase compared to the cipralex group, with no significant difference between control and cipralex plus SIL groups. SIL, silymarin. |
Table 3
| Islets of Langerhans parameter |
Group I (control) n=15 |
Group II (cipralex) n=15 |
Group III (cipralex plus SIL) n=15 |
Test | P-value |
|---|---|---|---|---|---|
| Number of islets of Langerhans | 3.50* | <0.001a | |||
| 1.87±0.74 | 0.67±0.73 | 1.40±0.51 | 1.79* | 0.07b | |
| 1–3 | 0–2 | 1–2 | 2.78* | 0.009c | |
| Diameter of islets of Langerhans | 5.54** | <0.001a | |||
| 263.4±21.87 | 217.0±23.96 | 246.33±35.38 | 1.59** | 0.12b | |
| 230–290 | 180–240 | 180–320 | 2.66** | 0.01c |
Table 4
| Collagen fiber deposition parameter |
Group I (control) n=15 |
Group II (cipralex) n=15 |
Group III (cipralex plus SIL) n=15 |
t-test | P-value |
|---|---|---|---|---|---|
| Area percentage of collagen fiber deposition | 19.46 | <0.001a | |||
| 9.56 ±2.36 | 25.70 ±2.17 | 10.81±1.24 | 1.81 | 0.08b | |
| 6.45–14.58 | 21.69–29.34 | 9.0–13.54 | 23.04 | <0.001c |
Table 5
| Immunohistochemical marker parameter |
Group I (control) n=15 |
Group II (cipralex) n=15 |
Group III (cipralex plus SIL) n=15 |
Test | P-value |
|---|---|---|---|---|---|
| iNOS | 4.67* | <0.001a | |||
| 6.11±2.84 | 25.05±7.11 | 8.38±2.99 | 1.76* | 0.08b | |
| 0.7–9.8 | 14.5–40.2 | 3.7–15.6 | 4.63* | <0.001c | |
| TNF-α | 4.67* | <0.001a | |||
| 5.30±4.24 | 30.93±6.46 | 8.19±3.47 | 1.81* | 0.07b | |
| 0.15–13.2 | 22–42 | 2.5–13.2 | 4.67* | <0.001c | |
| Caspase 3 | 4.67* | <0.001a | |||
| 5.25±2.78 | 45.87±9.11 | 7.03±2.91 | 1.64* | 0.10b | |
| 1.2–11.4 | 22.8–57.8 | 2.2–12.5 | 4.67* | <0.001c | |
| PCNA | 16.52** | <0.001a | |||
| 9.82±2.49 | 4.40±1.16 | 7.36±0.71 | 1.71** | 0.09b | |
| 4.5–14.9 | 2.5–6.2 | 6.1–8.9 | 15.73** | <0.001c | |
| Anti-insulin antibody | 5.23** | <0.001a | |||
| 43.15±7.08 | 32.68±3.14 | 38.69±5.83 | 1.88** | 0.07b | |
| 30.9–54.2 | 27.8–40.2 | 25.5–45.4 | 3.52** | 0.002c |
Values are presented as mean±SD or range. SIL, silymarin; iNOS, inducible nitric oxide synthase; TNF-α, tumour necrosis factor-alpha; PCNA, proliferating cell nuclear antigen. aComparison of group I and group II. bComparison of group I and group III. cComparison of group II and group III. *Mann Whitney U-test, **t-test.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download