Abstract
Choriocarcinoma occurs mainly in the gonads, but an extragonadal origin has been reported, albeit infrequently. Primary hepatic choriocarcinoma (PHC) is a rare malignancy, with only 11 cases reported. Most cases reported were in males, with none reported in pregnant females. A 23-year-old primigravida presented with a large liver lesion involving the right lobe of the liver at 28 weeks of pregnancy. Preoperative imaging was suggestive of hepatocellular carcinoma. She underwent a non-anatomical resection of the liver lesion. Surprisingly, her postoperative histopathology revealed a diagnosis of PHC. Her blood workup showed elevated beta human chorionic gonadotrophin. She underwent a termination of her pregnancy at 32 weeks. Before initiating adjuvant chemotherapy four weeks after surgery, a whole-body PET scan revealed multiple bi-lobar liver and pelvic deposits. After a multidisciplinary team discussion, she was started on adjuvant chemotherapy. She is currently under regular follow-up, seven months post-surgery. PHC, one of the vascular lesions of the liver, poses a diagnostic and therapeutic challenge, warranting a multidisciplinary approach.
Choriocarcinoma is a rare germ cell tumor with high malignant potential, which comes under the spectrum of gestational trophoblastic diseases.1 Gestational choriocarcinoma has a reported incidence of one in 20,000 in western countries.2 The incidence decreases after a non-molar pregnancy.3 Primary hepatic choriocarcinoma (PHC) involves the liver without a detectable lesion in the gonads, retroperitoneum, mediastinum, bladder, or prostate.4 Only 11 cases of PHC have been reported in the English literature, but none of them are in a pregnant female.1 This paper reports the first case of PHC in a female who presented during the third trimester of pregnancy.
As this is a case report, Nizam’s Institute Ethical Committee has confirmed that no ethical approval is required. Informed consent was taken from the patient and patient’s informed consent was obtained regarding publishing their data and photographs.
A 23-year-old primigravida, at 28 weeks of gestation, presented with pain in the right hypochondrium for one month without any other associated symptoms. Ultrasonography of the abdomen revealed a large heterogeneous, hyperechoic lesion in the right lobe of the liver. MRI of the abdomen showed an 11.6×10.2 cm-sized well-encapsulated exophytic mass lesion from the adjacent segments II, IV, and V with a large central scar. This scar was hypointense on the T1 weighted sequence and showed heterogeneous hyperintensity on the T2 weighted sequence (Fig. 1). The diffusion-weighted imaging showed a hyperintense signal along the periphery on high B value images with corresponding intermediate to low apparent diffusion coefficient (ADC). The central scar showed high ADC values. There was no signal drop on in-phase or opposed phases. No extrahepatic lesions were observed by imaging at the time of presentation. She had no risk factors for a hepatocellular carcinoma (HCC), such as a prior history of alcohol intake, and was negative for the hepatitis-b and hepatitis-c viruses. Her blood workup showed AFP of 117.8 ng/mL and serum ALP of 209 U/L with normal liver (total bilirubin: 0.5 mg/dL, AST: 32 IU/L, ALT 16 IU/L, PT-INR: 1.03) and renal function tests. The antenatal scan showed a live viable fetus with a gestational age of 28 weeks. With the patient and her family actively participating in decision-making, the decision to undergo surgery was made at the multidisciplinary tumor (MDT) board meeting. She was started on tocolytics, and all precautions were taken for emergency termination of her pregnancy if required. She underwent non-anatomical resection of the liver containing the tumor with a minimum 1 cm margin. The fetal monitoring was performed throughout her stay in the hospital. She had an uneventful recovery. A histopathological examination revealed an infiltrating lesion in the liver with large areas of necrosis and hemorrhage (Fig. 2). The cells were arranged in sheets and showed bizarre giant cells with frequent atypical mitoses. Some of the giant cells resembled syncytiotrophoblasts. The adjacent liver parenchyma was unremarkable. The tumor was immune-negative for the hepatocyte-specific antigen (has), Arginase, and Glypican-3, ruling out a primary HCC. On the other hand, the tumor cells showed intense immune expression for Sal-like protein 4, placental alkaline phosphatase, and beta-human chorionic gonadotropin (HCG), confirming the diagnosis of choriocarcinoma (Fig. 3). The histopathology and additional immunohistochemical stains (CD30, D2-40, CD117) did not reveal any other type of germ cell tumor, and it was diagnosed as a PHC.
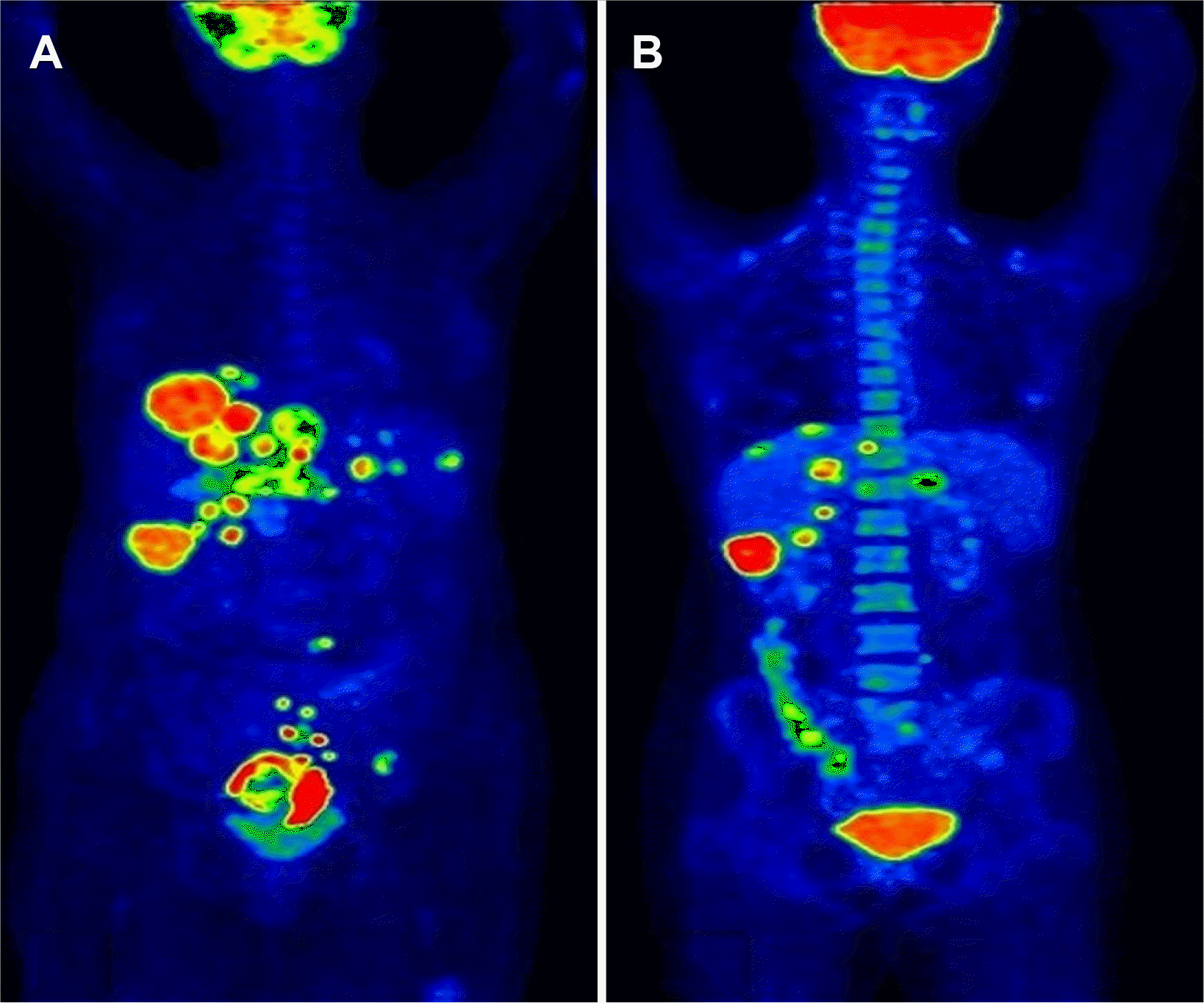
The patient’s condition was again discussed in MDT and planned for early elective termination of pregnancy. Therefore, an elective cesarean section was performed at 32 weeks of gestation. The premature baby died on the second day due to respiratory distress. The post-delivery beta-HCG and AFP levels were 11,875 IU/mL and 105 ng/mL, respectively. The histopathological examination of the placenta showed no evidence of choriocarcinoma. In the metastatic workup, a PET scan showed multiple scattered bi-lobar hepatic metastasis and pulmonary, presacral, rectal, and iliac nodal metastasis. No tumor was present in any gonadal site (Fig. 4A). After the multidisciplinary tumor board meeting, she was started on etoposide, methotrextate, actinomycin D, and cisplatin (EMA-EP regimen). The PET scan after six cycles of chemotherapy revealed the following: partial regression in the size, number, metabolism of hepatic lesions; reduced left iliac, presacral, and gluteal deposits; complete regression of right lung nodule (Fig. 4B). She is currently doing well and under regular follow-up seven months after the primary surgery.
PHC is extremely rare, with only 11 cases reported.1 Fernández Alonso et al. first reported the disease in 1992.5 Most cases of PHC were reported in males, with one reported in a non-gestational post-menopausal female. To the best of the authors’ knowledge, there are no reports of PHC in a pregnant female. The median age of PHC in these studies was 48 years. On the other hand, the present case was presented at a younger age. The most common symptoms were abdomen pain, abdominal lump, and tumor bleeding.6 The gross and histopathology of the present tumor were similar to the published literature.1,7,8 The crucial point was to exclude the other components of germ cell tumor and primary HCC. The absence of septal lymphocytes, Schiller Duval bodies, and derivatives of the germ cell layers ruled out an additional component of seminoma, yolk sac tumor, and teratoma, respectively.
The immunohistochemistry for these patterns was also negative in the present case. The minor elevation in AFP could be attributed to the pregnancy state because the morphology and the immunohistochemical stain did not reveal a yolk sac tumor. In the absence of pathognomonic clinical symptoms and radiological features, the preoperative suspicion of choriocarcinoma was extremely low. No gonadal or placental lesions were present on PET computed tomography and histopathological examination of the placenta.
Primary choriocarcinoma is a rare tumor identified in the gonads, mediastinum, lung, brain, and digestive tract. Most of the published literature has reported this tumor in men. Jiang et al.7 reported the largest series of 13 males with primary choriocarcinoma at various locations. There are no case reports of primary choriocarcinoma like this in a pregnant female. Several hypotheses have been postulated for the origin of these tumors in extragonadal locations. Dedifferentiation of primary adenocarcinoma, development from displaced gonadal anlage or teratoma, and transformation of primary liver adenoma have been proposed.8-10 The cytogenetics of these tumors need to be determined and compared with the primary tumors of those sites to confirm these hypotheses.
Thus far, surgery followed by chemotherapy is the treatment of choice.8 On the other hand, choriocarcinoma is an aggressive tumor with a high propensity for early hematogenous metastasis, as evident in the present case because she developed a distant metastasis within four weeks of the complete resection of the tumor.1,6 They carry a dismal prognosis with reported overall survival of two–eight months.6 The diagnosis of extragonadal choriocarcinoma was confirmed after excluding the possibility of tumor or tumor scars in the gonads at autopsy in the reported literature.8 Nevertheless, it might not be feasible in all scenarios, so the criteria should be comprehensive based on the available resources for live patients
In conclusion, PHC can be considered one of the differential diagnoses for vascular lesions of the liver. The diagnosis and management of PHC, especially in a pregnant patient, is a challenging task that requires a multidisciplinary approach.
ACKNOWLEDGEMENTS
Dr. Madhur Kumar Srivastava from the Department of Nuclear Medicine for providing the images related to the case report.
REFERENCES
1. Kohler A, Welsch T, Sturm AK, et al. 2018; Primary choriocarcinoma of the liver: a rare, but important differential diagnosis of liver lesions. J Surg Case Rep. 2018:rjy068. DOI: 10.1093/jscr/rjy110.

2. Yousefi Z, Saied S, Davachi B, Rezaei A, Mirzamarjani F. Postmenopausal choriocarcinoma: case report and lliterature review. Int J Cancer Manag. 2017; Mar. 20. doi: 10.5812/ijcm.4400. DOI: 10.5812/ijcm.4400.
3. Lee E, Cho H. 2019; A case of intraplacental choriocarcinoma with pulmonary metastasis. Case Rep Oncol. 12:802–806. DOI: 10.1159/000503816. PMID: 31762752. PMCID: PMC6873010.

4. Ahn Y, Kim JH, Park CS, Kim TE, Hwang S, Lee SG. 2018; Multidisciplinary approach for treatment of primary hepatic choriocarcinoma in adult male patient. Ann Hepatobiliary Pancreat Surg. 22:164–168. DOI: 10.14701/ahbps.2018.22.2.164. PMID: 29896579. PMCID: PMC5981148.

5. Sekine R, Hyodo M, Kojima M, et al. 2013; Primary hepatic choriocarcinoma in a 49-year-old man: report of a case. World J Gastroenterol. 19:9485–9489. DOI: 10.3748/wjg.v19.i48.9485. PMID: 24409080. PMCID: PMC3882426.

6. Shi H, Cao D, Wei L, Sun L, Guo A. 2010; Primary choriocarcinoma of the liver: a clinicopathological study of five cases in males. Virchows Arch. 456:65–70. DOI: 10.1007/s00428-009-0864-1. PMID: 20013345.

7. Jiang F, Xiang Y, Feng FZ, Ren T, Cui ZM, Wan XR. 2014; Clinical analysis of 13 males with primary choriocarcinoma and review of the literature. Onco Targets Ther. 7:1135–1141. DOI: 10.2147/OTT.S62561. PMID: 25018640. PMCID: PMC4074184.

8. Martins VF, Moreno F, Vizcaíno JR, Santos J. 2015; Primary gastric choriocarcinoma: A rare case. Int J Surg Case Rep. 14:44–47. DOI: 10.1016/j.ijscr.2015.07.009. PMID: 26218175. PMCID: PMC4573415.

9. Xiong Y, Yang MX. 2019; Primary gastric choriocarcinoma with multiple metastases - A case report and literature review of carcinogenesis. Hum Pathol Case Rep. 18:200330. DOI: 10.1016/j.ehpc.2019.200330.

10. Qiu J, Jia S, Li G. 2018; Incidence and prognosis factors of extragonadal choriocarcinoma in males: a population-based study. Cancer Manag Res. 10:4565–4573. DOI: 10.2147/CMAR.S175948. PMID: 30410393. PMCID: PMC6197831.

Fig. 1
(A) Coronal MRI images of the abdomen showing a well-encapsulated exophytic liver lesion (white arrow) with a large central scar (asterisk) and fetus (arrowhead). (B) Axial (T2) sections of the exophytic liver lesion with central scar (asterisk).
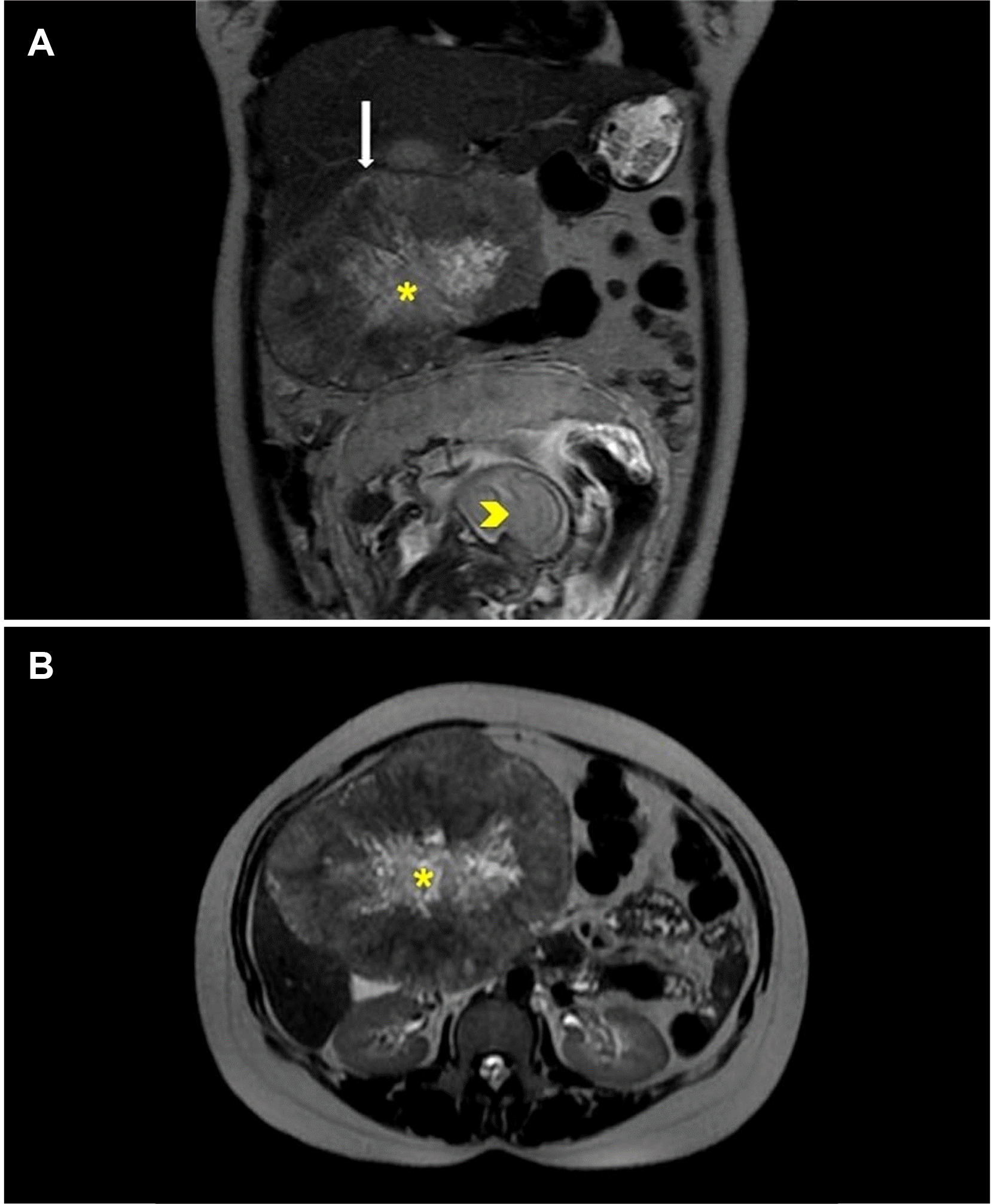
Fig. 2
Photomicrographs showing: (A) tumor cells infiltrating liver parenchyma (normal liver- black arrow). H&E ×40. (B) Infiltrating tumor. H&E ×100. (C) Scattered syncytiotrophoblastic cells (black arrowheads), highlighted within the inset. H&E ×400. (D) Mitotic activity with atypical mitoses (within the white circle), abnormal tumor cell (black arrow). H&E ×400.
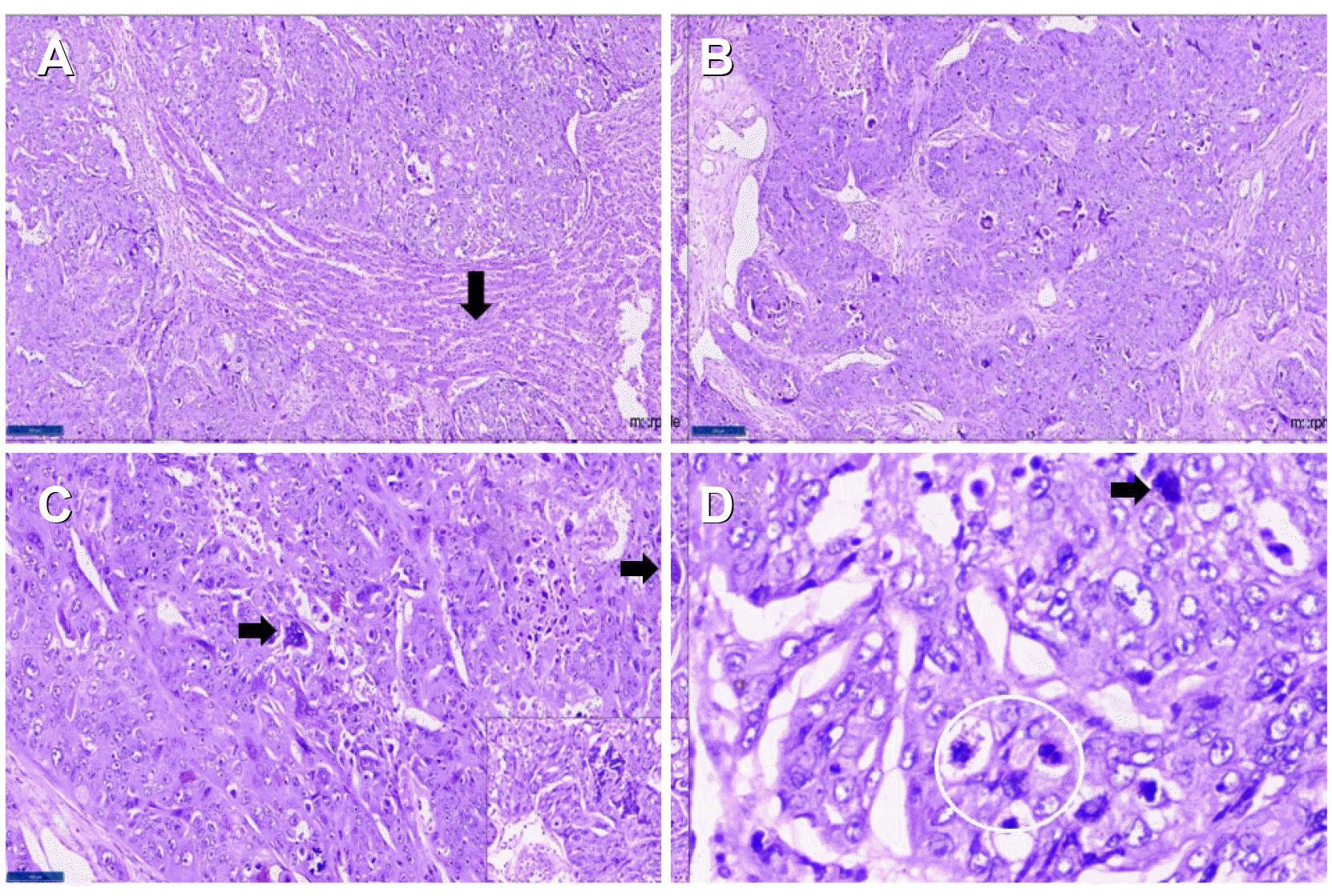
Fig. 3
Immunohistochemical panel showed: (Top panel) (A) Positive Sal-like protein-4 (SALL-4); horseradish peroxidase (HRP) Polymer ×100. (B) Cytoplasmic Beta Human Chorionic Gonadotrophin (HCG); HRP Polymer ×100. (C) Positive Pan cytokeratin (pan CK); HRP Polymer ×100. (Bottom panel) The tumor cells were immuno-negative for (D) hepatocyte-specific antigen (HSA) (control hepatocytes positive) (E) Arginase, and (F) D2-40; HRP Polymer ×100.

Fig. 4
PET scan (four weeks after surgery) pre-chemotherapy showing: (A) Multiple 18-fluoro deoxyglucose (FDG) avid lesions noted in both lobes of the liver and perirectal nodules. (B) Post six cycles of chemotherapy showing partial regression in size, number, metabolism of metabolism of hepatic lesions, left iliac, presacral, and gluteal deposits, and complete regression of right lung nodule.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download