Introduction
Periodontitis is a chronic inflammation that occurs in deep periodontal tissues and can lead to attachment loss, alveolar bone resorption, and eventually tooth loss (
1,
2). Bone regeneration is the main objective of periodontal therapy. Tissue engineering has recently provided alternative technologies to regenerate periodontal bone tissues by transplanting appropriate cells and scaffolds (
3,
4). Stem cells from human exfoliated deciduous teeth (SHED) are a type of mesenchymal stem cells with strong osteogenic and proangiogenic properties (
5-
7). When combined with various scaffolds, SHED have the potential to enhance bone defect repair (
8-
10). However, little is known about the cellular and molecular mechanisms underlying SHED promotion of efficient bone repair. The question of whether a direct action through differentiation of the implanted cells or a paracrine role through growth factor secretion remains unresolved (
10,
11).
Cell tracking
in vivo is an important method to study the molecular mechanisms by which transplanted cells promote bone regeneration. In this study, magnetite (Fe
2O
3)-based superparamagnetic iron oxide nanoparticles (SPIOs) were used as the labeling agents for magnetic resonance imaging (MRI) cell tracking. Transplanted areas containing SPIO-labeled cells will appear as regions of low signal intensity, creating negative contrast on T2 weighted MRI (
12). However, previous research has suggested that SPIO particles may be phagocytized by macrophages, resulting in the division and death of labeled cells as well as the formation of false-positive MRI results (
13). Fluo-rescence-labeled SPIO, such as Molday ION Rhodamine-B
TM (MIRB), can easily be detected and tracked by fluorescence microscopy and confirmed by MRI scans, making it a superior SPIO-labeled material (
14). This technology not only allows real-time identification of transplanted stem cells using MRI but also allows detection of transplanted cells using histochemistry (
15). A high dose of MIRB is toxic to cells and inhibits cell proliferation and differentiation; however, a low MIRB dose will influence the MRI tracer signal. Consequently, we must establish an optimal labeling concentration of MIRB by analyzing the proliferation and differentiation of SHED labeled with various MIRB concentrations (
16).
MIRB concentration was set at 25 μg Fe/ml in this investigation because it not only satisfied the signal intensity of MRI detection but also did not have a negative impact on cell proliferation and differentiation. MRI, HE staining, and immunohistochemistry results indicated that both labeled and unlabeled SHED transplanted to the periodontal bone defect region could promote bone rege-neration. The colocalization of MIRB and hNUC showed that the transplanted SHED could survive in vivo. Immu-nofluorescence and enzyme-linked immunosorbent assay (ELISA) revealed that SHED could not only differentiate into osteoblast-like and osteocyte-like cells but it could also secrete osteogenic proteins to mobilize host cells. These findings suggested that SHED might stimulate bone repair through both direct cell differentiation and indirect paracrine processes.
Go to :

Materials and Methods
Cell culture and osteoinductive treatment
Deciduous teeth were extracted from healthy children aged 6∼8 years following a procedure approved by the Ethics Committee of Liaocheng People’s Hospital (Liaocheng, China). SHED were isolated from the dental pulp using an outgrowth method as described in a previous report (
17). For osteoinductive treatment, cells were seeded at a density of 2×10
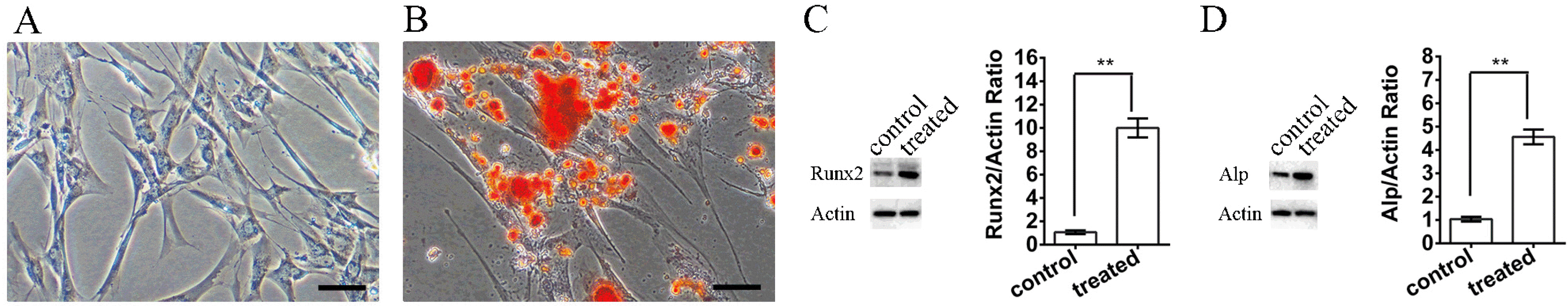
5 cells/well in a six-well plate and cultured in an osteoinductive medium for 2 weeks. The medium was replaced twice a week. The osteoinductive medium was composed of the following ingredients: 90% DMEM (Gibco, Carlsbad, CA, USA), 10% fetal bovine serum (Gibco, Carlsbad, CA, USA), 100 U/ml penicillin (Gibco, Carlsbad, CA, USA), 100 U/ml streptomycin (Gibco, Carlsbad, CA, USA), 100 nmol/l dexamethasone, 10 mM β‑glycerol phosphate, 50 nM vitamin D3, and 50 μM l-ascorbic acid 2-phosphate (Sigma‑Aldrich, St. Louis, Missouri, USA). Alizarin Red S staining was used to detect the mineral deposit 2 weeks after osteogenic induc-tion. Cells were fixed with 4% paraformaldehyde (m/v; Sigma-Aldrich, St. Louis, Missouri, USA) and then stai-ned with 40 mM Alizarin Red S (pH 4.2; Sigma-Aldrich, St. Louis, Missouri, USA) for 10 min at room temperature. Cells were observed, and images were captured with an inverted fluorescence microscope (Nikon Ti, Tokyo, Japan).
Western blot analysis
Total protein was isolated from the osteogenic induction group and non-treated group with RIPA buffer and quantified with a BCA protein assay kit (Beyotime, Beijing, China). Equal quantities of samples were separated via 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a hydrophi-lic polyvinylidene fluoride (PVDF) membrane (0.45 μm, Merck Millipore, Boston, MA, USA) with the Bio-Rad protein assay system (Bio-Rad, Hercules, CA, USA). Primary Antibodies against Runx2 (1:1,000, Abcam, Cambridge, UK), Alp (1:1,000, Abcam, Cambridge, UK), and β-ac-tin (1:2,000, Santa Cruz Biotechnology, CA, USA) were incubated with the membrane at 4℃ overnight. The secondary antibodies, horseradish peroxidase (HRP)-linked goat anti-rabbit IgG or goat anti-mouse IgG (Santa Cruz Biotechnology, CA, USA), were incubated with the membrane at room temperature for 1 h. The membrane was immersed with electrochemiluminescence (ECL) response solution (Pierce, Waltham, MA, USA) at room temperature for 1 min. All bands were analyzed with Image Lab Software (version 5.1; Bio-Rad Laboratories, CA, USA), and relative levels against the internal control β-actin were calculated.
Fluorescence observation of MIRB-labeled SHED
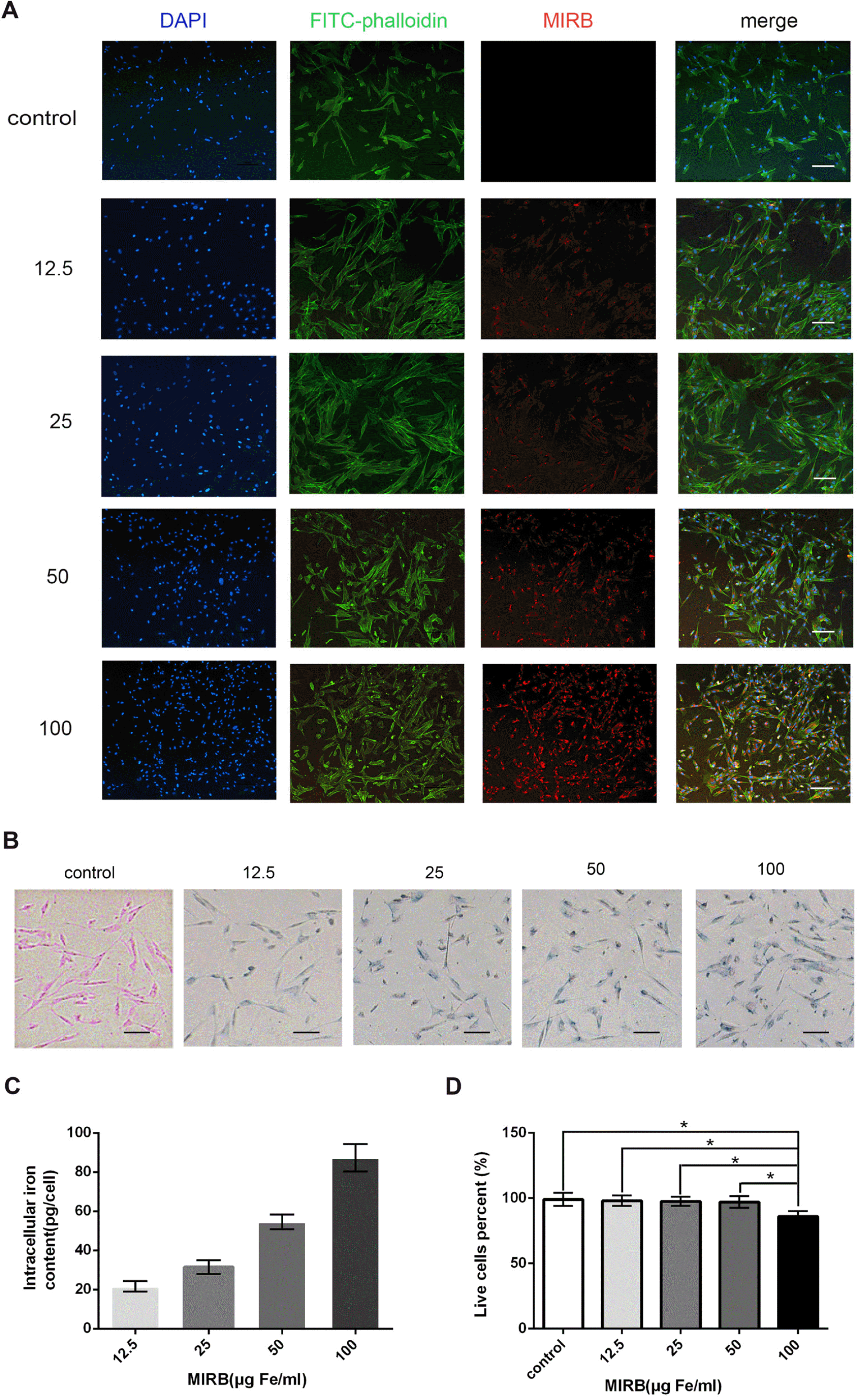
Cells were seeded in six-well plates at a density of 2×105 cells/well. MIRB (BioPAL Co., Worcester, MA, USA) stock solution (2 mg Fe/ml) was added to cell culture medium (DMEM+10% FBS) to prepare labeling medium with final concentrations of 0, 12.5, 25, 50, and 100 μg Fe/ml. SHED were then incubated in the labeling medium for 24 h under standard culture conditions (37℃, 5% CO2, humidified). Cells were washed three times with PBS and fixed with 4% paraformaldehyde for 15 min. After washing the cells three times with PBS, these were incubated with a blocking solution (5% BSA+1% Triton X-100 in PBS) for 1 h. A FITC-phalloidin conjugate solution (Enzo, New York, NY, USA) was then used to stain filamentous actin at room temperature for 1 h. The cells were again washed three times with PBS and then observed under a fluorescence microscope.
Prussian blue staining and labeling efficiency calculation
SHED were labeled with various MIRB concentrations, and then fixed with 4% paraformaldehyde. SHED were washed three times with PBS and then stained with Perl’s Prussian blue reagent (2% potassium ferrocyanide in 6% hydrochloric acid) (Leagene Biotechnology, Beijing, China) for 30 min. The intracellular distribution of Fe3+ was observed under a light microscope. In general, we selected 10 fields of view randomly to count Prussian blue positive stained cells under a 10-fold microscope. The labeling efficiency was determined using the following equation: Labeling efficiency (%)=(number of Prussian blue positive cells/number of total cells)×100%.
Detection of intracellular iron content
The average intracellular iron concentration was determined using an iron assay kit (Biovision, Inc., CA, USA). Cells were plated in six-well plates at a density of 2×105 cells/well and incubated with a MIRB labeling medium for 24 h. Labeled SHED were then lysed with 65 μl of Iron Assay Buffer. The lysate was centrifuged at 16,000×g for 10 min. A 50 μl volume of supernatant was added to each well of a 96-well plate, and an equivalent amount of Iron Assay Buffer was added to create a total volume of 100 μl. A 5 μl iron reducer was added to each well to convert Fe3+ to Fe2+. A 100 μl of iron probe was added to each well and incubated for 60 min at room temperature. The absorbance was read with a spectrometer at 593 nm. Mean while, an iron content standard curve was created according to the kit instruction. The total iron content in each well was determined according to the standard curve. The number of cells in each well was counted with a hemocytometer. The iron content was determined using the following formula: The iron content of a single cell=Total iron content per well/Number of cells per well.
Trypan blue staining and viable cell percentage calculation
We prepared a 4% Trypan blue stock reagent by dissolving 4 g of Trypan blue (Merck KGaA, Darmstadt, Hessen, Germany) in 100 ml of double-distilled water and filtering the solution through a filter paper. The stock reagent was diluted to 0.4% with PBS before use. A single-cell suspension was prepared from five groups of cells labeled with 0, 12, 25, 50, and 100 μg Fe/ml MIRB, respectively. An equal amount of 0.4% Trypan blue and single-cell suspension were mixed, and the mixture was incubated for approximately 3 minutes at room temperature. Then, live and dead cells were counted individually under a microscope (dead cells were stained light blue and live cells were colorless). The percentage of viable cells was computed using the following formula: Live cells percent (%)=Total number of live cells/(Total number of live cells+Total number of dead cells)×100%.
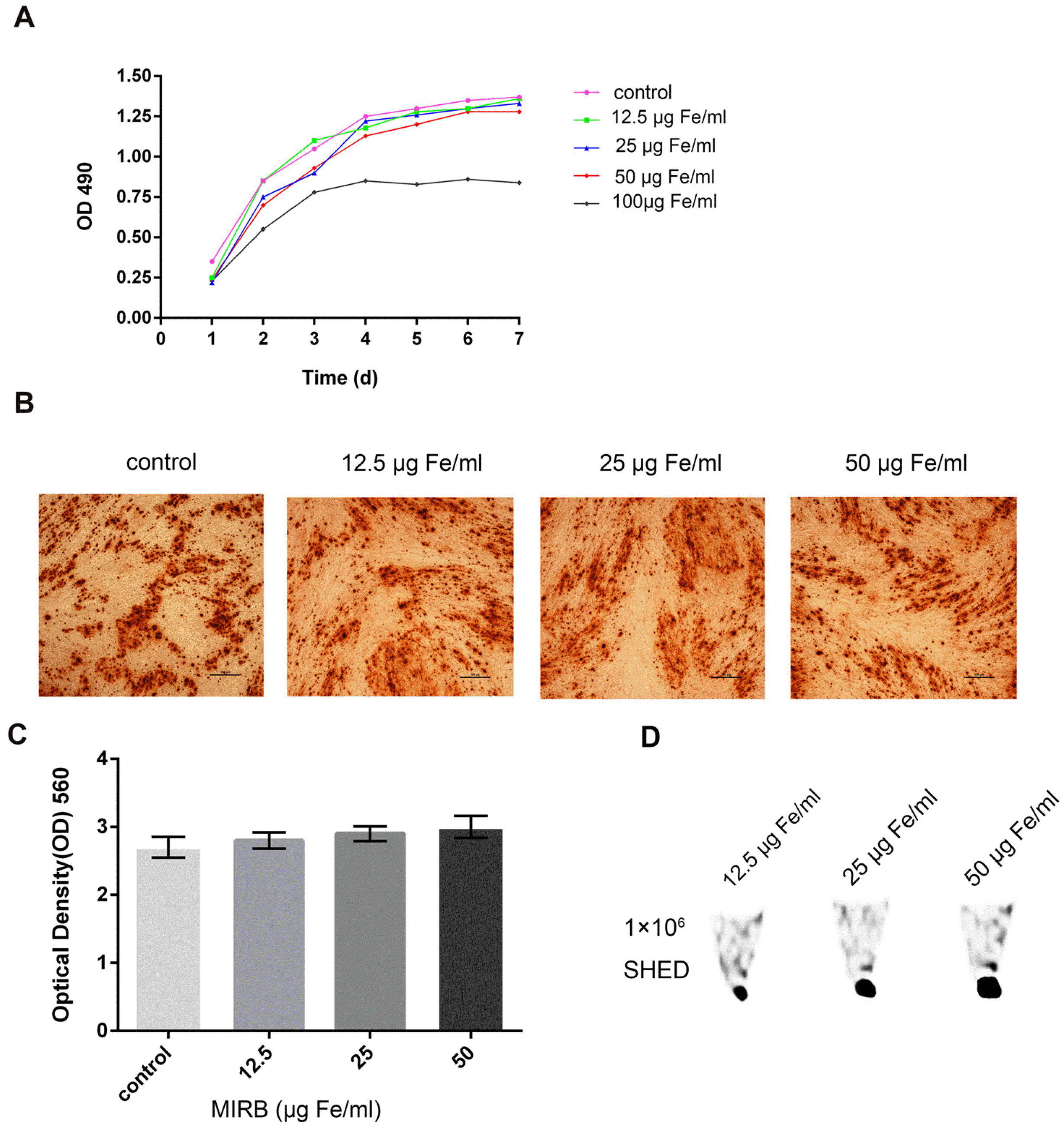
Effect of MIRB labeling on the proliferation of SHED
SHED were seeded in a 96-well plate at a density of 1,000 cells/well. Once the SHED adhered to the wall of the wells, they were incubated with a labeling medium containing various concentrations of MIRB (0, 12.5, 25, 50, and 100 μg Fe/ml) for 24 h. After that, the labeling medium was replaced with 100 μl of a standard culture medium and the SHED were cultured for an additional 1, 3, 5, and 7 d. At each time point, 10 μl of CCK-8 solution (Yeasen, Shanghai, China) was added to each well of the plate and the mixture was incubated at 37℃ for 2 h. Finally, absorbance at 450 nm was determined using a microplate spectrophotometer (Bio-Rad, CA, USA).
Effects of MIRB labeling on the osteogenic differentiation of SHED
SHED were seeded in a six-well plate at a density of 2×105 cells/well. The cells were incubated with a labeling medium containing different concentrations of MIRB (0, 12.5, 25, and 50 μg Fe/ml) for 24 h. Labeled SHED were then cultured in an osteoinductive medium for 2 weeks. The cells were fixed with 4% paraformaldehyde and stained with 2 ml of Alizarin Red S solution (pH 4.2; Sigma‑Aldrich, St. Louis, Missouri, USA) for 15 min. Images were captured using an inverted fluorescence microscope. For semi-quantitative analysis of Alizarin Red S, 600 μl of cetylpyridinium chloride solution (100 g/l; Sigma‑Aldrich, St. Louis, Missouri, USA) was added to each well and incubated for 15 min at room temperature. Absorbance was read using a spectrometer at 562 nm (Bio-Rad, CA, USA).
MRI analysis of MIRB labeled SHED in vitro
SHED labeled with different concentrations of MIRB (0, 12.5, 25, and 50 μg Fe/ml) were collected and counted with a hemocytometer. One million cells from each group were transferred to1.5-ml centrifuge tubes (Eppendorf, Westbury, NY, USA). After centrifugation at 150×g for 5 min, SHED were resuspended in 15 μl of thrombin solution. Then, the 15 μl of thrombin solution containing 1×106 cells and 15 μl of fibrinogen solution were simultaneously injected into the bottom of the 1.5-ml centrifuge tube. The mixture condensed to jelly in 1∼2 min. The tubes were then imaged on a 1.5-T system (Achieva, Philips Healthcare, Best, The Netherlands) with an eight-channel wrist coil (repetition time [TR]=20 ms, International time [TE]=8.1 ms, flip angle=25, field of view [FOV]=80×80×30 mm3, slice thickness=4 mm, and Mat=208×208).
Animals and group design
All animal experiments were performed following a protocol approved by the Animal Committee of Liaocheng People’s Hospital (Liaocheng, China). Forty-eight SD rats (6∼8 weeks old, 200∼250 g, male) were numbered and divided randomly into four groups: (
1) SHED (MIRB), (
2) SHED, (
3) Fibrin, and (
4) PBS. Each rat has a defect in the right mandible. Six rats from each group were anesthetized at 0, 3, 6, and 9 weeks after the surgery. The healing of the defect was captured by MRI. At 2 and 4 weeks after the surgery, three rats in each group were perfused to obtain samples of periodontal bone defects for histological and immunohistochemical analyses.
Preparation of periodontal bone defects in rats
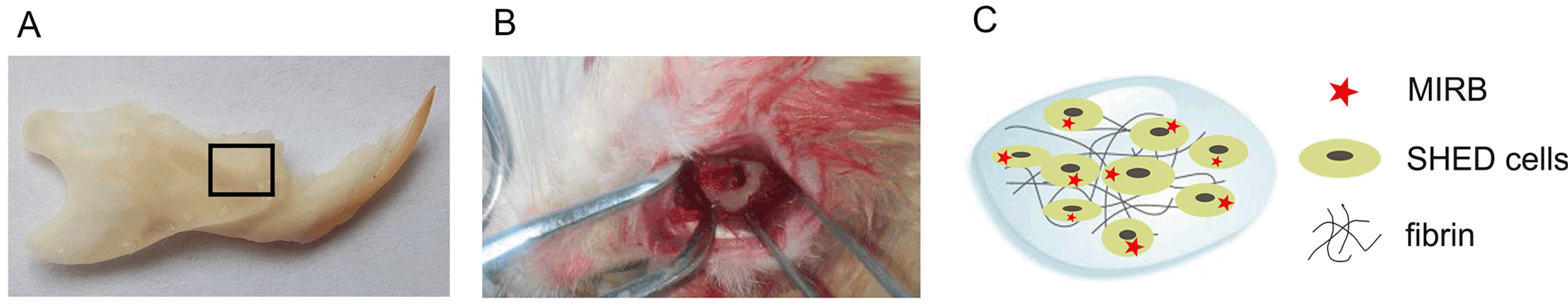
Periodontal bone defects were constructed according to the following procedures. The rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (4 ml/kg). An approximately 2 cm long extraoral incision was made parallel to the lower border of the rats’ mandibles. The subcutaneous tissues and masseter muscles were separated from the alveolar bone surface. A periodontal wound defect (length×height×depth: 5 mm×4 mm×1 mm) was constructed with a low-speed dental round bur, accompanied by physiological saline irrigation. The defect was located at 1 mm behind the anterior mandible and 1 mm below the crest of the alveolar bone. The muscle and skin were sutured, and the wound was cleaned with iodophor after surgery. To prevent the immune system from rejecting the transplanted cells, we intraperitoneally injected cyclosporine A (10 mg/kg; Sigma-Aldrich, St. Louis, Missouri, USA) into the rats 24 h before transplantation and every day after the surgery until the animals were euthanized.
Preparation of fibrin glue containing SHED for transplantation
Fibrin glue was prepared with a fibrin sealant kit (SHANGHAI RASS, Shanghai, China). One million fresh SHED (MIRB-labeled or unlabeled) were resuspended in a 15 μl thrombin solution (450 IU/ml) for cell trans-plantation. The thrombin solution containing SHED (labeled or unlabeled) was injected into the defect sites of rats. Simultaneously, an equal amount of fibrinogen solution (40 mg/ml) was injected into the same site. In the rats in the Fibrin group, both thrombin and fibrinogen solutions were injected into the defect site, whereas in the rats in the PBS group, only PBS was injected.
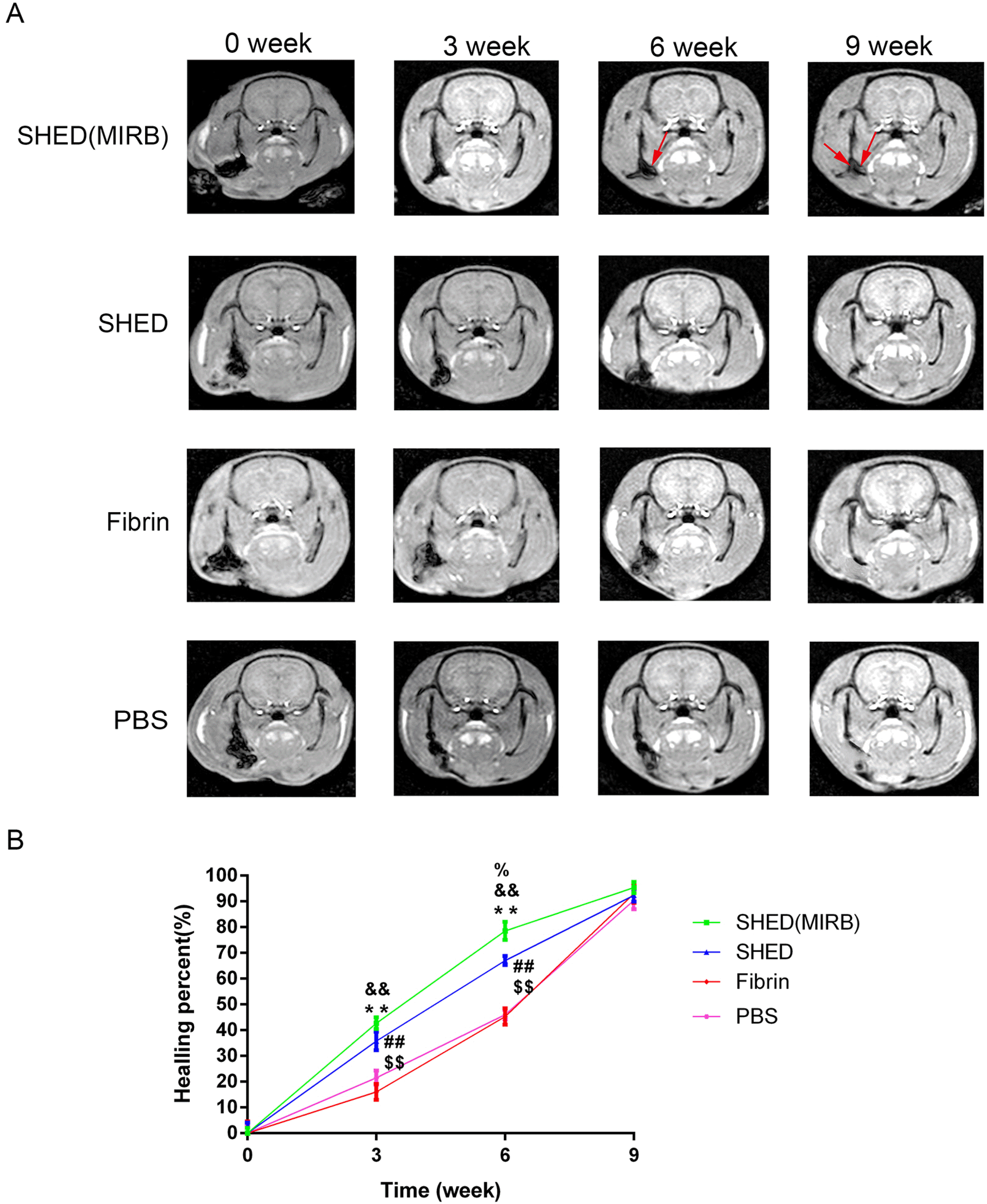
MRI evaluation and analysis after transplantation of SHED into rats
MRI analysis on rats was performed on a 1.5-T system (Achieva, Philips Healthcare, Best, The Netherlands) with an eight-channel wrist coil at 0, 3, 6, and 9 weeks after the surgery. Subjects were positioned face down in the prone position. Three-dimensional axial and coronal fast-field echo T1-weighted imaging “black bone” sequences were scanned.
The obtained DICOM files were reconstructed in an axial and coronal plane for image analysis, with recon-struction layers of 40 and a thickness of 0.5 mm. Qua-litative and quantitative evaluations were performed by two board-certified radiologists. Imaging parameters included defect area in defined regions of interest and signal intensity value.
Harvesting and preparation of samples for histological and immunohistochemical examination
The animals were sacrificed at 2 and 4 weeks after the surgery. Rats were anesthetized with 10% chloral hydrate (4 ml/kg), perfused with 200 ml of normal saline, and then fixed with 200 ml of 4% paraformaldehyde in phosphate buffer. The right mandibles of each rat were excised and fixed with 4% PFA for 24 h. The mandibles were decalcified in 10% Ethylene Diamine Tetraacetie Acid (EDTA) at room temperature for 4 weeks. The decalcification solution was replaced twice a week. After complete decalcification, the bone tissues were dehydrated with gradient ethanol and immersed in paraffin for 2 h. The tissues were immersed in paraffin and sliced into 3- to 4-μm-thick sections. These paraffin sections were heated in a 62℃ oven for 20 min for HE or 2 h for immunohistochemical staining.
Histological staining, immunohistochemical staining, and immunofluorescence staining
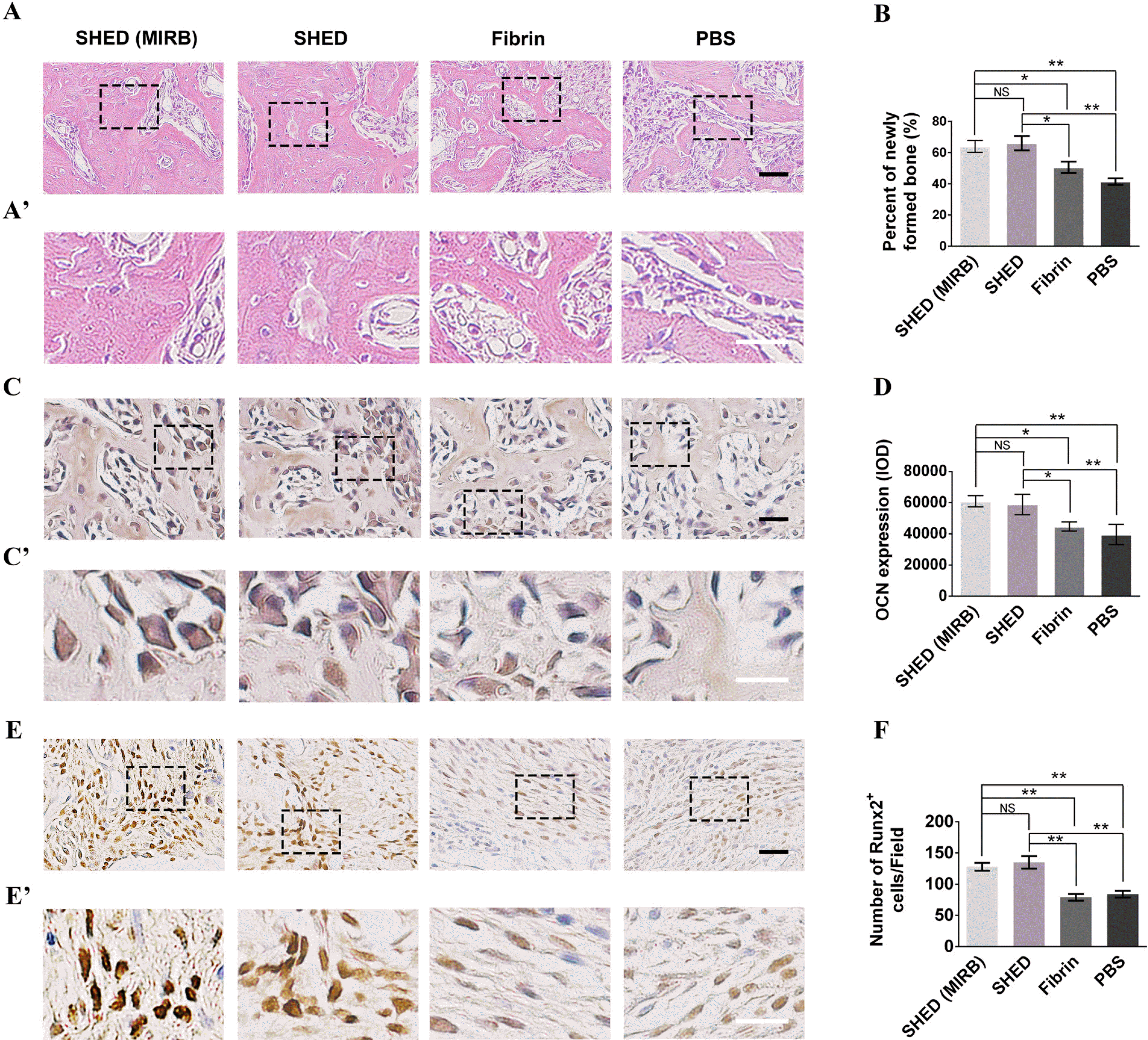
Every fifth section of each group was stained with a hematoxylin and eosin kit (Beyotime, Shanghai, China). Samples were observed under a Ti microscope (Nikon, Tokyo, Japan) and measured using Image-Proplus 6.0 software (Media Cybernetics, Silver Spring, MD, USA). The percentage of newly formed bones was determined using the following formula: Newly formed bone (%)=(area of newly formed bone/area of the total defect)×100%.
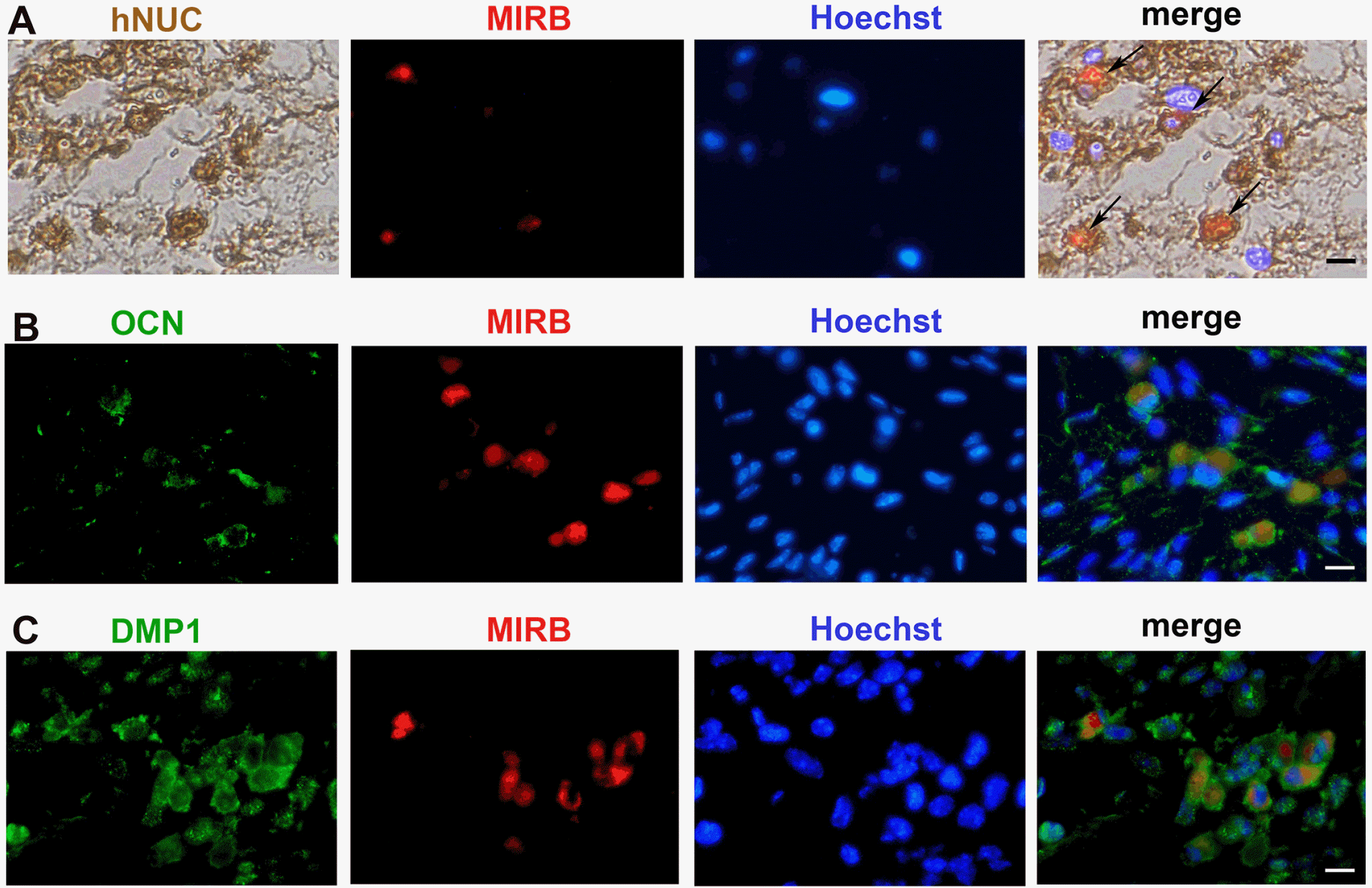
Immunohistochemical staining was performed using the Biotin-Streptavidin HRP Detection System (ZSGB-BIO, Beijing, China) according to the kit instruction. Primary antibodies against hNUC (1:200), Runx2 (1:1,000), and OCN (1:500) were purchased from Abcam (Abcam, Cambridge, UK). Control sections were incubated with PBS instead of primary antibodies. Immunoreaction was detected with a diaminobenzidine (DAB) substrate kit (Maxim Biotechnology Co., Ltd., Fuzhou, China). Images were taken on a microscope (Nikon, Tokyo, Japan). The integrated optical density of Runx2 and OCN was calculated using the Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA).
Before performing immunofluorescence staining, the sections were deparaffinized and blocked with 5% goat serum for 1 h. The sections were then incubated with primary antibodies against DMP1 (1:500), OCN (1:500), Runx2 (1:500), and Col II (1:500, all from Abcam, Cambridge, UK) at 4℃ overnight. Control sections were treated with PBS instead of primary antibodies. Samples were incubated with fluorescent-labeled secondary antibodies (1:500, Abcam, Cambridge, UK) for 2 h. Colo-calization of MIRB with DMP1, OCN, Runx2, and Col II was observed using a fluorescence microscope (Nikon, Tokyo, Japan).
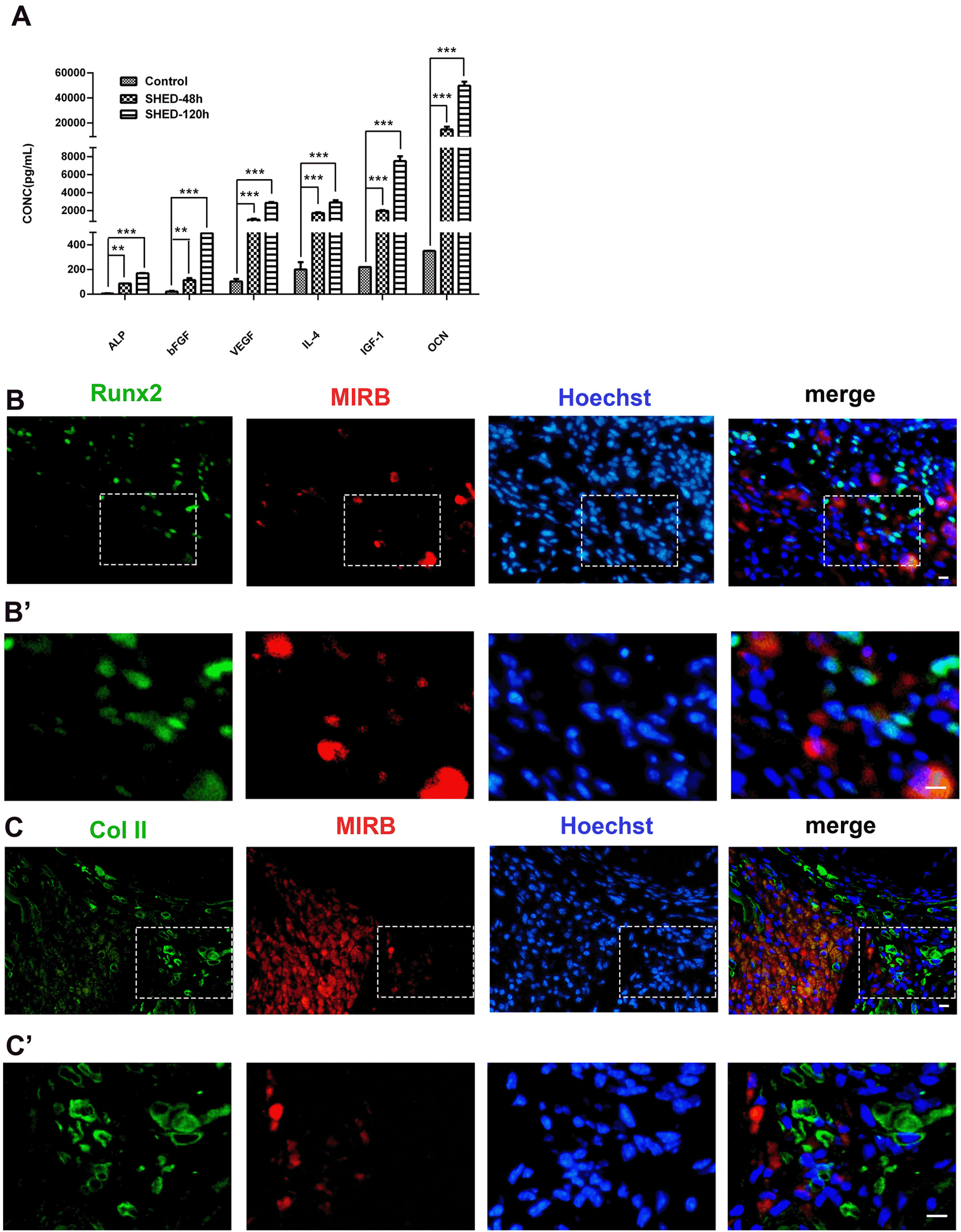
Detection of the cytokines secreted by SHED
Cells were seeded at a density of 2×105 cells/well in a six-well plate. The next day, the culture medium was changed to a serum-free medium. The serum-free medium was collected after incubation with SHED for 0, 48, and 120 h. SHED-secreted cytokines were detected with the ELISA kit (Shenggong Biotechnology, Shanghai, China) according to the manufacturer’s instructions. A total of 100 μl of sample or cytokine standard was added to a pre-coated 96-well plate and incubated for 1.5 h at 37℃. The liquid was discarded, and the plate was dried. A 100 μl working solution of biotin-conjugated antibodies was added to each reaction well and incubated for 60 min at 37℃. The plates were rinsed four times with 1×washing buffer. A total of 100 μl of HRP-conjugated streptavidin was added to each well and incubate for 30 min to form an immune complex. The plates were rinsed four times. A total of 90 μl of substrate reagent (hiding from light) was added to each reaction well. The color developed at 37℃ for about 15 min. 50 μl of a stop solution was added to each reaction well to stop the enzyme–substrate reaction. The OD value at a wavelength of 450 nm was measured immediately using a microplate reader (within 5 min). The results were obtained by removing blanks from the standards.
Statistical analysis
Data were expressed as mean±standard deviation, and all data were analyzed using SPSS software (SPSS Inc., IL, USA). Comparisons between multiple groups were performed using ANOVA, and comparisons between two groups were performed using an independent-samples t-test. The statistical probability of p<0.05 was considered significant.
Go to :

Discussion
Alveolar bone destruction is a common hallmark of human periodontitis (
18). In this study, we tried to transplant SHED into rats to repair the periodontal bone defect. The results showed that both MIRB-labeled and unlabeled SHED could promote bone tissue regeneration. MRI analysis revealed that MIRB-labeled SHED could be tracked for 9 weeks
in vivo. Immunofluorescence staining showed that some SHED could differentiate into osteoblast-like and osteocyte-like cells. However, most SHED did not differentiate or migrate
in vivo. In addition, a large number of endogenous rat pre-osteoblasts and chondrocytes gathered in the peripheral areas of the transplanted cells. SHED could release osteogenesis-related substances such as OCN, ALP, IGF-1, IL-4, VEGF, and bFGF to alter the microenvironment of the transplanted area. These findings implied that SHED promotes bone repair through direct cell differentiation and paracrine mobilization of endogenous rat cells.
Stem cell tracking is an important method for revealing the mechanism of stem cell therapy by indicating the distribution and differentiation of transplanted cells (
19). Many tracking methods, including MRI, fluorescence ima-ging, and ultrasound imaging have been developed (
20). In this study, we used MRI to track cells
in vivo by labeling SHED with a SPIO negative contrast agent. A new SPIO agent Molday ION Rhodamine-B (MIRB) was used to track the transplanted SHED. MIRB is labeled with Rhodamine B, a fluorescent dye, and can be visualized by both MRI and fluorescence imaging (
15,
16,
21,
22). However, as a direct cell labeling approach that relies on the accumulation of nanoparticles in the cytoplasm, MIRB has some technical flaws. First, high concentrations of intracellular free iron are toxic to the cells. Our results indicated that MIRB concentrations more than 100 μg Fe/ml had a negative impact on cell viability and proliferation, which is in line with previous research (
16). A MIRB concentration of 25 μg Fe/ml was employed to fulfill not only the signal intensity required for MRI detection but also to have no detrimental effect on cell proliferation and differentiation. Second, the labeled nanoparticles will be lost during cell division, resulting in weakening of the MRI detection signal (
23). At 9 weeks after the surgery, MRI images revealed that the rat’s periodontal bone defect had healed completely, but there were still low-density artifacts at the transplant site produced by MIRB. Previous research found that the MIRB-labeled signal can last for 60 d and 42 weeks in different studies, but the MRI signal gradually fades over time (
16,
24). This demonstrates that, although MIRB is lost to varying degrees after transplantation, it can still be used as a reference for the survival of transplanted cells
in vivo. Third, apoptosis and macrophage phagocytosis create leakage of MIRB particles from labeled cells, which are readily absorbed by nearby host cells, resulting in false-positive results (
20). To determine whether the low-density artifact was generated by MIRB-labeled SHED or MIRB overflow-labeled rats’ cells, we performed immunohistochemical staining with the human cell-specific antigen hNUC. The findings revealed that all cells with red MIRB fluorescence were positive for hNUC histochemistry, not all hNUC-positive cells had red MIRB fluorescence. This demonstrated that MIRB was lost with cell proliferation after cell transplantation
in vivo. However, a significant number of transplanted cells were still labeled with MIRB, and MIRB tracking can still reflect the survival of transplanted cells
in vivo.
In recent years, stem cell transplantation has emerged as one of the most promising approaches for bone regeneration therapy (
25). However, the stem cell transplantation mechanism for bone regeneration therapy remains questionable. There are two main points of view. The first is that stem cell transplantation repairs bone defects through direct differentiation, whereas the second is that stem cells promote bone healing through paracrine pathways (
26). Previous findings have proven that the transplanted cells could differentiate into osteocytes and deposit bone matrix proteins such as OPN and OCN to induce new bone formation in the grafted defect area (
27). Our study revealed that transplanted SHED can differentiate into OCN-expressing osteoblast-like cells and DMP1-expressing osteocyte-like cells, implying that the transplanted cells can participate in bone healing through direct differentiation. However, a large number of Runx2- and Col II-positive cells were discovered around the transplanted cells. Runx2 is a pre-osteoblast marker and a critical regulator in the early stages of bone repair (
28). Col II is a hypertrophic chondrocyte marker and a key player in the endochondral ossification process during bone repair (
29). The transplanted SHED attracted large numbers of host pre-osteoblasts and hypertrophic chondrocytes, suggesting that the transplanted SHED can mobilize the host cells through paracrine to promote bone healing. These findings suggested that SHED promoted bone repair via direct differentiation and paracrine processes.
In the future, we should focus on deciphering the signaling pathways governing SHED destiny selection after implantation, as well as the molecular dialog between SHED and host cells in vivo. Determining the mechanisms by which SHED repair the bone defect, whether through direct differentiation or paracrine pathway, is indeed essential for future progress in tissue engineering strategies.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download