Abstract
Background and Objectives
mRNA-based protein expression technology has been used to express functional proteins. We have previously generated dopamine neurons from rat-embryo derived neural precursor cells (NPCs) through repeated transfection of synthetic transcription factor mRNA encoding dopamine-inducible genes. However, NPCs began to die approximately 10 d post-transfection. In this study, we examined a long-term transfection protocol that did not affect cell viability.
Methods and Results
Experiments were performed in eight groups sorted according to the start date of mRNA transfection. mRNA was transfected into NPCs daily for 21 d and live cell images of each group were recorded. NPCs which were differentiated for more than five days showed sustained gene expression and appreciable viability despite daily mRNA transfection for 21 d.
Various techniques for exogenous protein expression have been widely used in both research and medicine. DNA-based gene expression using viral and non-viral vectors is most commonly used to express functional proteins in cells (1). However, DNA-based approaches have several drawbacks, including integration into the host genome and increased immunogenicity.
Recently, synthetic mRNA-based protein expression has emerged as an attractive alternative for DNA-based gene expression (2). The strategy is based on non-viral technology and has an advantage in terms of safety because mRNA itself does not enter the nucleus or integrate into the host genome (3, 4). In addition, mRNA-mediated protein expression works in both mitotic and post-mitotic cells, in contrast to DNA-mediated gene expression (5). Furthermore, delivery of multiple mRNAs in one step together makes it possible to express multiple genes stoichiometrically (6). Despite these advantages over DNA-based expression, the technique still has concerns with respect to stability and immunogenicity given that mRNA is prone to rapid degradation within the cytoplasm due to its unstable structure, and introduction of mRNA into cells can induce the interferon pathway.
We have previously successfully generated dopaminergic (DA) neurons from neural precursor cells (NPCs) by tran-sfection of mRNAs encoding the Nurr1 and FoxA2 genes (7). However, the mRNA-based differentiation of DA neurons requires daily transfection of dopamine-inducible mRNAs because of the short half-life of mRNA. Repeated mRNA transfection was essential for the stable expression of NURR1 and FOXA2 proteins. Unfortunately, undesi-rable cytotoxicity in NPCs occurs during daily and repea-ted transfections of mRNA, suggesting that transfecting large amounts of mRNA resulted in cytotoxicity (7).
In this study, we examined whether temporal control of mRNA transfection affects the cytotoxicity of NPCs. Pre- or post-differentiated NPCs were subjected to daily mRNA transfection for 21 d. We found that the initial transfection of mRNA after 5 d of differentiation showed notably low cytotoxicity in NPCs. Together, these data suggest that cytotoxicity can be reduced by temporal control of mRNA transfection.
The animals were housed and treated according to the Institutional Animal Care and Use Committee (IACUC 2021-0065) guidelines of Hanyang University, Korea. Rat NPCs were obtained from the brain cortical tissues of Sprague–Dawley rat embryos (embryonic day 14) (Daehan Biolink, Seoul, Korea). Rat NPCs were cultured in dishes coated with 15 μg/ml poly-L-ornithine (PLO; Sigma-Al-drich, St Louis, MO, USA) and 1 μg/ml fibronectin (FN; Sigma-Aldrich) and incubated at 37℃ and 5% CO2. To enable expansion of rat NPCs, the cells were cultured in a N2 medium supplemented with 20 ng/ml basic fibroblast growth factor (bFGF, R&D Systems, Minneapolis, MN, USA). For neuronal differentiation, the cells were cultured in a N2 medium supplemented with 0.2 mM ascorbic acid (Sigma-Aldrich), 20 ng/ml brain-derived neurotrophic factor (R&D Systems), 20 ng/ml glial cell-derived neurotrophic factor (R&D Systems), and 250 μg/ml dibutyryl-cAMP (Sigma-Aldrich).
The pcDNA/UTR55A vector for mRNA synthesis was prepared by modifying the pcDNA3.1(+) plasmid (Invi-trogen, Carlsbad, CA, USA). Several restriction enzyme sites (896∼930 and 980∼992 bp) of pcDNA3.1(+) were replaced with multiple cloning sites (NheI, BamHI, NotI, and XbaI). Oligonucleotide sequences of the 5’-untran-slated region (UTR) forward, 5’-UTR reverse, 3’-UTR forward, 3’-UTR reverse, 55pA forward, and 55pA reverse (Integrated DNA Technologies, Coralville, IA, USA) were annealed and inserted into the pcDNA3.1(+). Enhanced green fluorescent portein gene (EGFP) was inserted between the 5’- and 3’-UTR of pcDNA/UTR55A as a reporter of successful transfection and gene expression.
RNA was synthesized as previously described (7). The construct encoding EGFP mRNA was linearized using EcoRV (Takara Bio, Shiga, Japan). RNA was prepared using the MEGAscript T7 kit (Ambion, Austin, TX, USA), with the digested construct as a template. To prepare the final construct, we applied 5’ capping and poly (A) tailing to the RNA using the ScriptCap m7G capping system, 2’-O-methyltransferase, and poly (A) polymerase kit (CELLS-CRIPT, Madison, WI, USA). Finally, mRNA was precipitated with 2.5 M ammonium acetate (Ambion) and dissolved in an RNA Storage Solution (Ambion). EGFP mRNA was stored at −70℃ at a concentration of 1 mg/ml (Fig. 1).
mRNA transfection was performed as previously described (7). One day before transfection, NPCs (50,000 cells/Ø12 mm) were seeded onto glass slides in 24-well plates coated with PLO/FN, and incubated in an expansion medium. Synthetic mRNA and transfection reagent Lipofectamine 2000 (Invitrogen) were separately diluted in an Opti-Minimal Essential Medium (Opti-MEM; Invitrogen) and incubated for five minutes at room temperature, after which the two Opti-MEM samples were mixed and incubated at room temperature for 20 min. The 24-well plates seeded with mouse NPCs were washed with Opti-MEM, and fresh Opti-MEM was added to the wells. The mRNA-lipofectamine mixture was then added to the wells. After three hours, the solutions in the transfected wells were replaced with expansion medium.
The NPCs were seeded into a PLO/FN-coated 24-well plate and divided into several groups. Each group consist-ed of three wells and was classified according to transfec-tion initiation day. Live cells were recorded daily using a Nikon camera (D5600) and microscope (Eclipse Ta2-FL) for 21 d, and Kaplan–Meier survival curves were generated.
The mRNA encoding EGFP was used to monitor gene expression (Fig. 1). NPCs harvested from rat embryonic brains on embryonic day 14 were expanded in the N2 medium supplemented with bFGF for 2 d. After 2 d, the NPCs were subjected to differentiation into neuronal cells in the N2 medium without bFGF. The cultured cells were divided into eight groups, as indicated in Table 1, and subjected to daily transfection for 21 d (Fig. 2). The EGFP expression and cell viability were evaluated daily. Cells from groups 1 to 6 showed high lethality after the 10th transfection (Fig. 3, Supplementary Fig. S1∼S3). However, significant cytotoxicity was not observed in groups 7 and 8 even after the 21st transfection, suggesting that the start of mRNA transfection at the late differentiation period can reduce cytotoxicity due to repetitive mRNA loads (Fig. 3B).
Genetic diseases, which are mostly incurable, include all illnesses caused by abnormal changes in a patient’s genes. Gene therapy replaces a mutated gene or adds a new gene to either produce a therapeutic effect, or to treat the disease. Gene therapy has been demonstrated to be useful for treating genetic diseases and cancers through fusion of genes with therapeutic effects (8-10).
Gene transfer using viral vectors, including lentiviruses and adeno-associated viruses, has effective gene expression capability (11-13). However, target genes encoded by viral vectors have the potential to be randomly integrated into the recipient’s chromosomes because of their viral nature. Considering these safety issues, we chose mRNA as the non-viral vector for gene delivery. A gene transfer system using mRNA is safer than using viral vectors (4, 14), but the system has some disadvantages, such as low gene transfer efficiency and rapid degradation of mRNA vectors (15).
To prevent the rapid degradation of mRNA within 1∼2 d, we performed daily transfection of mRNA encoding a specific gene to induce long-term expression. Using this met-hod, the NPCs were converted into dopaminergic cells. When mRNA transfection was performed daily, early transfected NPCs did not survive in the long term, whereas NPCs differentiated for more than seven days maintained their gene expression under normal conditions for 21 d with daily transfection (7).
In the present study, we investigated the duration to which NPCs must be differentiated to enable the long-term expression of genes without affecting cell conditions during daily mRNA transfection. An mRNA vector encoding EGFP was synthesized and used for the convenient observation of gene expression. The transfection start date varied according to the initial differentiation period, with 21 transfections conducted daily in triplicate. NPCs differentiated for fewer than five days exhibited cell death after the 10th transfection. NPCs differentiated for more than six days remained stable throughout all 21 transfections, with strong long-term EGFP expression.
Although further confirmation from different cell types is required, this phenomenon may be a stabilizing effect according to neuron formation and differentiation of astrocytes from NPCs. NPCs and neurons are vulnerable to external stimuli, but their stability increases in the presence of astrocytes and supporting cells (16). When NPCs are cultured in neuron-conditioned medium (NCM), the cells differentiate into TUJ1-positive neurons and GFAP-positive astrocytes (17, 18). In our experiment, an increase in the number of astrocytes was also observed during NPC culture with NCM (Supplementary Fig. S3).
Daily mRNA transfection is considered as an unbearable level of damage in these undifferentiated NPCs, possibly resulting in cell death. When NPCs are subjected to a sufficient differentiation period, the cells may be maintained in stable conditions with astrocyte support.
Our study provides a foundation for the further analysis of daily mRNA transfection to achieve continuous gene expression for gene therapy.
Supplementary data including three figures can be found with this article online at https://doi.org/10.15283/ijsc22125.
References
1. Sung YK, Kim SW. 2019; Recent advances in the development of gene delivery systems. Biomater Res. 23:8. DOI: 10.1186/s40824-019-0156-z. PMID: 30915230. PMCID: PMC6417261. PMID: bf6a6ce2d576497899d4a5004c821aee.
2. Sahin U, Karikó K, Türeci Ö. 2014; mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 13:759–780. DOI: 10.1038/nrd4278. PMID: 25233993.
3. McLenachan S, Zhang D, Palomo AB, Edel MJ, Chen FK. 2013; mRNA transfection of mouse and human neural stem cell cultures. PLoS One. 8:e83596. DOI: 10.1371/journal.pone.0083596. PMID: 24386231. PMCID: PMC3873397. PMID: 691578f40808496b910b2f0f0c0b4bc2.
4. Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, DeVine A, Ettenger A, Fitzgerald K, Godfrey M, Gupta D, McPherson J, Malwadkar P, Gupta M, Bell B, Doi A, Jung N, Li X, Lynes MS, Brookes E, Cherry AB, Demirbas D, Tsankov AM, Zon LI, Rubin LL, Feinberg AP, Meissner A, Cowan CA, Daley GQ. 2015; A comparison of non-integrating reprogramming methods. Nat Biotechnol. 33:58–63. DOI: 10.1038/nbt.3070. PMID: 25437882. PMCID: PMC4329913.
5. Yamamoto A, Kormann M, Rosenecker J, Rudolph C. 2009; Current prospects for mRNA gene delivery. Eur J Pharm Biopharm. 71:484–489. DOI: 10.1016/j.ejpb.2008.09.016. PMID: 18948192.
6. Mandal PK, Rossi DJ. 2013; Reprogramming human fibroblasts to pluripotency using modified mRNA. Nat Protoc. 8:568–582. DOI: 10.1038/nprot.2013.019. PMID: 23429718.
7. Kim SM, Lim MS, Lee EH, Jung SJ, Chung HY, Kim CH, Park CH. 2017; Efficient generation of dopamine neurons by synthetic transcription factor mRNAs. Mol Ther. 25:2028–2037. DOI: 10.1016/j.ymthe.2017.06.015. PMID: 28705346. PMCID: PMC5589083.
8. Bulaklak K, Gersbach CA. 2020; The once and future gene therapy. Nat Commun. 11:5820. DOI: 10.1038/s41467-020-19505-2. PMID: 33199717. PMCID: PMC7670458. PMID: e73be1499a3f43d1abd476b0fd01fb8c.
9. Giamas G, Gagliano T. 2022; Cancer gene therapy 2020: highlights from a challenging year. Cancer Gene Ther. 29:1–3. DOI: 10.1038/s41417-021-00340-6. PMID: 33963297. PMCID: PMC8103066.
10. Papanikolaou E, Bosio A. 2021; The promise and the hope of gene therapy. Front Genome Ed. 3:618346. DOI: 10.3389/fgeed.2021.618346. PMID: 34713249. PMCID: PMC8525363. PMID: 0150aea3236049339e98244cab643a0d.
11. Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. 2021; Viral vector platforms within the gene therapy landscape. Signal Trans-duct Target Ther. 6:53. DOI: 10.1038/s41392-021-00487-6. PMID: 33558455. PMCID: PMC7868676. PMID: 63666028eb554ff786d8586456529231.
12. Lundstrom K. 2018; Viral vectors in gene therapy. Diseases. 6:42. DOI: 10.3390/diseases6020042. PMID: 29883422. PMCID: PMC6023384.
13. Wang D, Tai PWL, Gao G. 2019; Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 18:358–378. DOI: 10.1038/s41573-019-0012-9. PMID: 30710128. PMCID: PMC6927556.
14. Huang CL, Leblond AL, Turner EC, Kumar AH, Martin K, Whelan D, O'Sullivan DM, Caplice NM. 2015; Synthetic chemically modified mrna-based delivery of cytoprotective factor promotes early cardiomyocyte survival post-acute myocardial infarction. Mol Pharm. 12:991–996. DOI: 10.1021/mp5006239. PMID: 25588055.
15. Zhou X, Hao R, Chen C, Su Z, Zhao L, Luo Z, Xie W. 2020; Rapid delivery of nanobodies/VHHs into living cells via expressing in vitro-transcribed mRNA. Mol Ther Methods Clin Dev. 17:401–408. DOI: 10.1016/j.omtm.2020.01.008. PMID: 32128345. PMCID: PMC7044678.
16. Wang XF, Cynader MS. 1999; Effects of astrocytes on neuronal attachment and survival shown in a serum-free co-culture system. Brain Res Brain Res Protoc. 4:209–216. DOI: 10.1016/S1385-299X(99)00019-7. PMID: 10446416.
17. Chang MY, Son H, Lee YS, Lee SH. 2003; Neurons and astrocytes secrete factors that cause stem cells to differentiate into neurons and astrocytes, respectively. Mol Cell Neurosci. 23:414–426. DOI: 10.1016/S1044-7431(03)00068-X. PMID: 12837625.
18. Wang FW, Hao HB, Zhao SD, Zhang YM, Liu Q, Liu HJ, Liu SM, Yuan QH, Bing LJ, Ling EA, Hao AJ. 2011; Roles of activated astrocyte in neural stem cell proliferation and differentiation. Stem Cell Res. 7:41–53. DOI: 10.1016/j.scr.2011.03.004. PMID: 21530437.
Fig. 1
Preparation of mRNA encoding EGFP. Prepared plasmid DNA of EGFP mRNA was linearized using the restriction enzyme EcoRV. Linearized DNA was used as a template for in vitro transcription. To prepare the final mRNA, a 5’-cap and poly(A) tail were added to the in vitro-transcribed RNA.
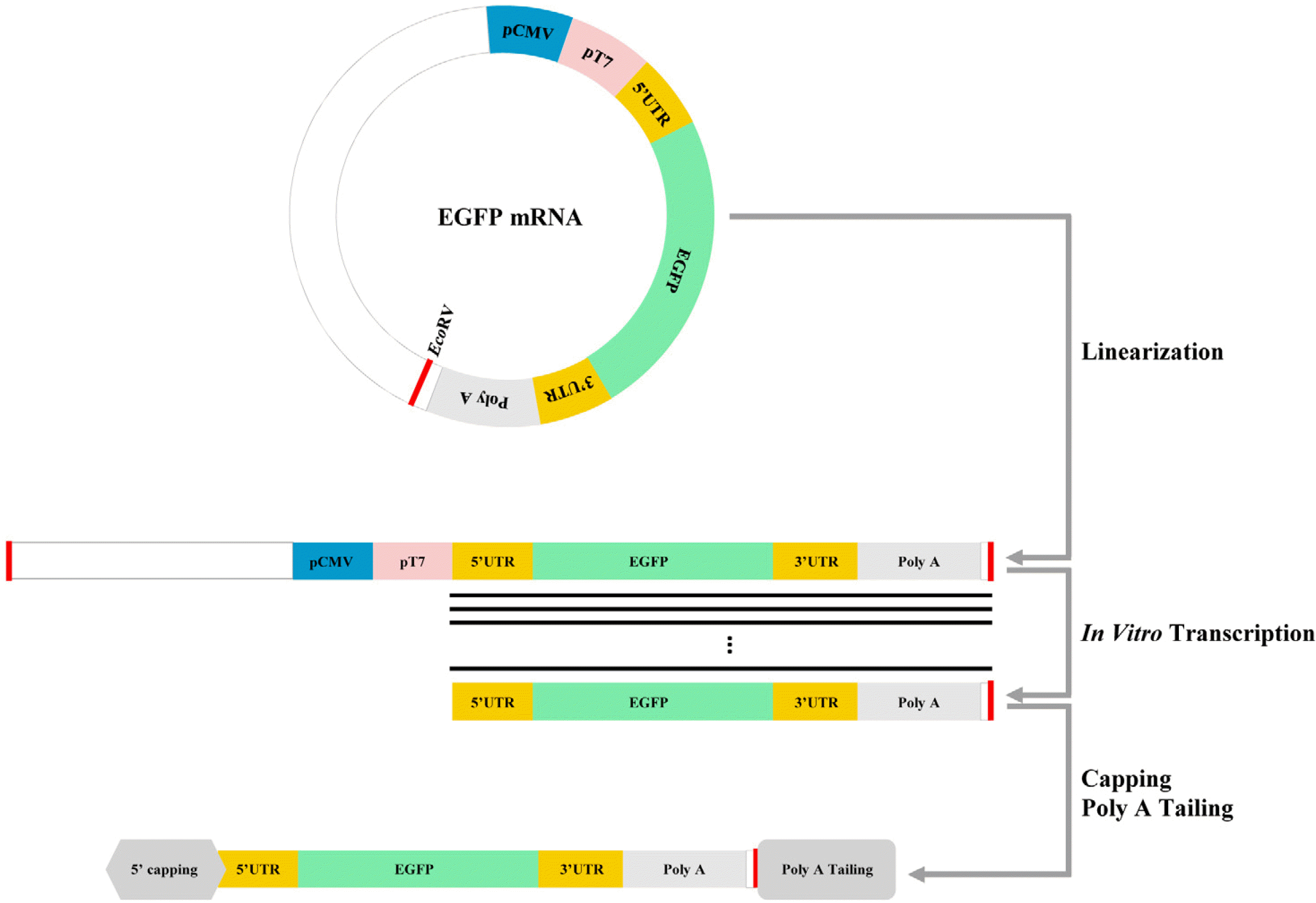
Fig. 2
Daily mRNA transfection experiment 1. The transfection start date varied according to the initial differentiation period. The neural precursor cells from each group were transfected with EGFP mRNA for 21 d.
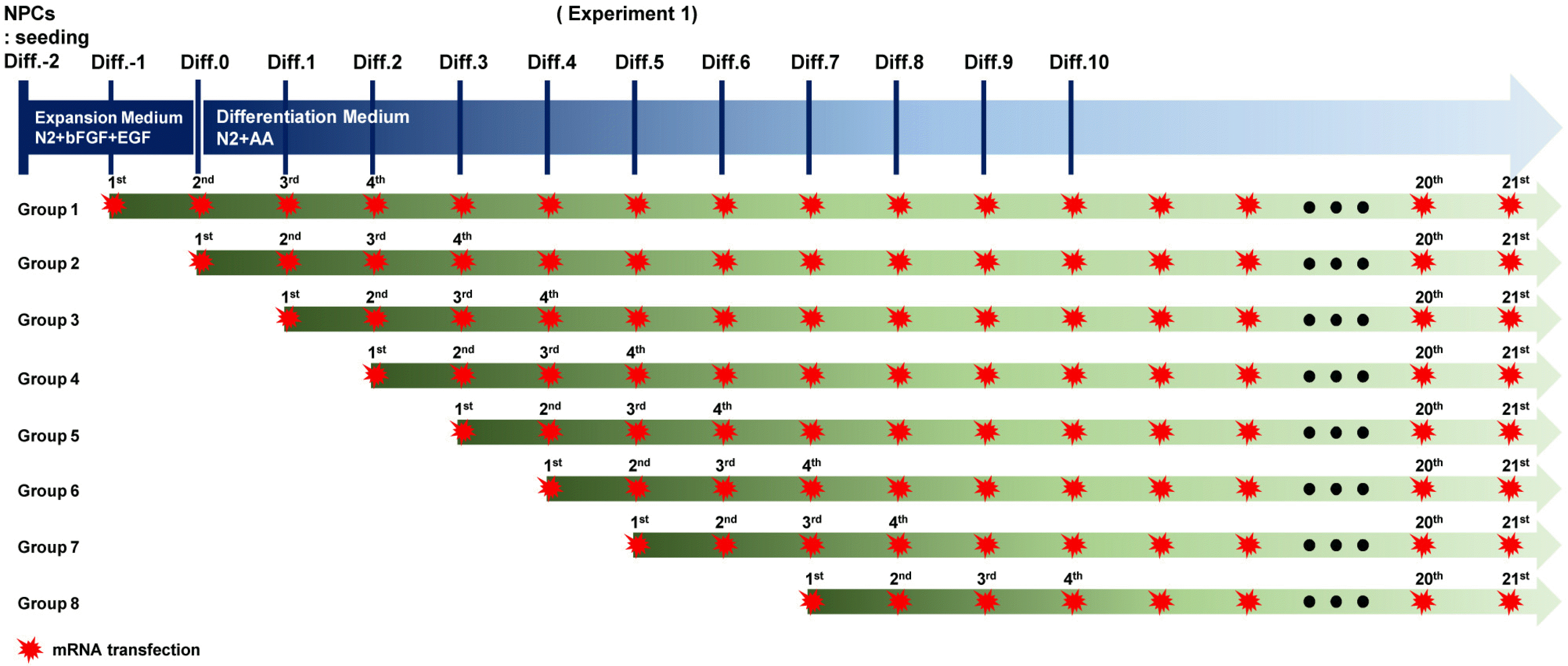
Fig. 3
Cell viability and live cell images following repeated mRNA transfection. Synthetic EGFP mRNA was transfected each day into the rat neural precursor cells. (A) Viability graph was prepared by quantifying the wells that died out of three wells for each group during daily mRNA transfection, and (B) EGFP-expressing live cells of two groups (groups 1 and 8) were recorded. The scale bar represents 100 μm.
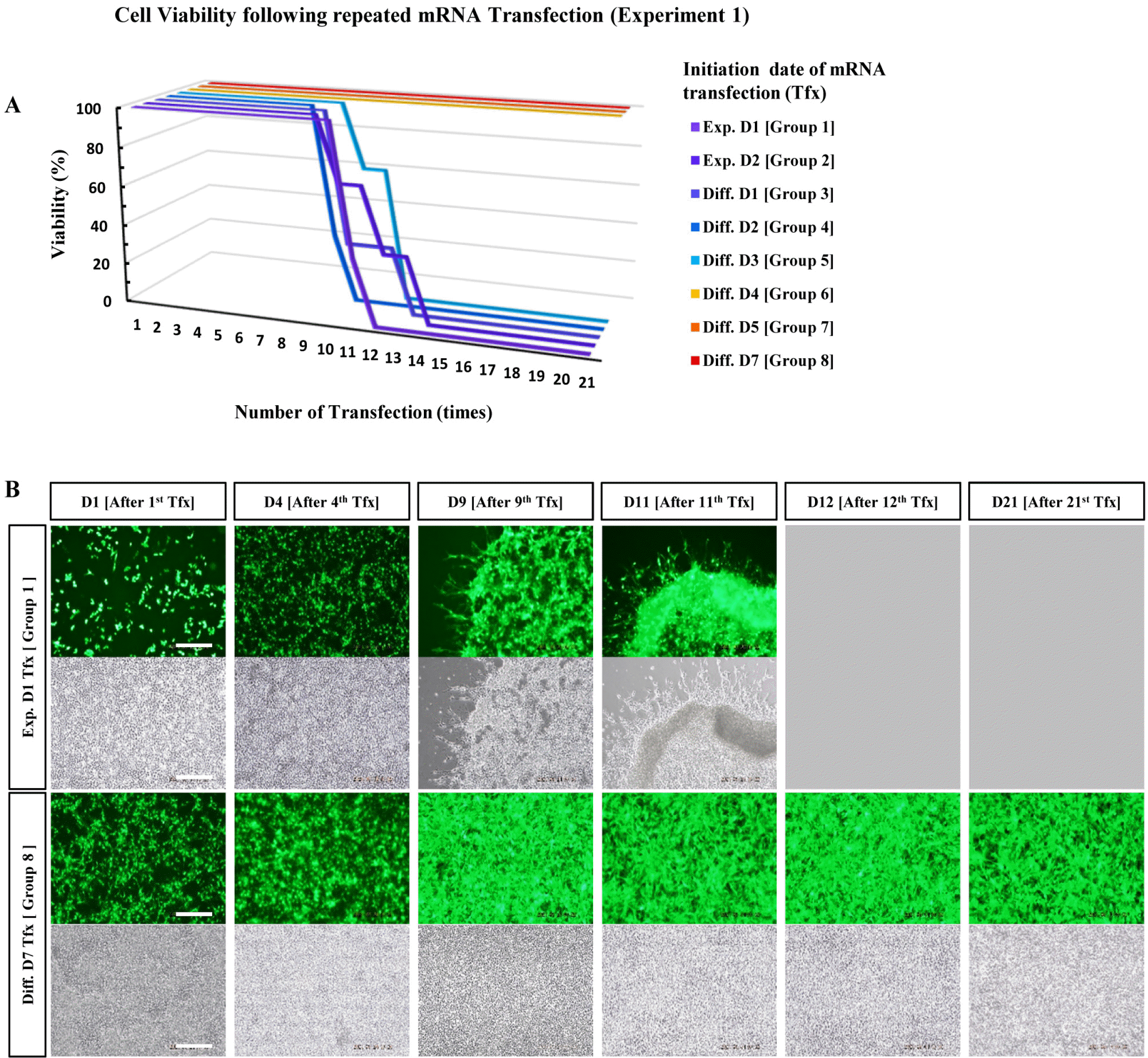
Table 1
Group classification




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download