Abstract
γδ T cells are a rare and unique prototype of T cells that share properties with natural killer cells in secondary lymphoid organs. Although many studies have revealed the function and importance of adult-derived γδ T cells in cancer biology and regenerative medicine, the low numbers of these cells hamper their application as therapeutic cell sources in the clinic. To solve this problem, pluripotent stem cell-derived γδ T cells are considered alternative cell sources; however, few studies have reported the generation of human pluripotent stem cell-derived γδ T cells. In the present study, we investigated whether lymphoid lineage γδ T cells were successfully generated from human pluripotent stem cells via hemogenic endothelium under defined culture conditions. Our results revealed that pluripotent stem cells successfully generated γδ T cells with an overall increase in transcriptional activity of lymphoid lineage genes and cytolytic factors, indicating the importance of the optimization of culture conditions in generating lymphoid lineage γδ T cells. We uncovered an initial step in differentiating γδ T cells that could be applied to basic and translational investigations in the field of cancer biology. Based on our result, we will develop an appropriate method to purify γδ T cells with functionality and it helpful for the study of basic mechanism of γδ T cells in pathophysiologic condition as well as clinic application.
Since the discovery of γδ T cells (1), significant progress has been made showing the importance of γδ T cells, which are a prototype of T cells, have emerged as cell sources for immunotherapy (2-4). The remarkable success of chimeric antigen receptor-expressing T (CAR)-T and CAR-natural killer (NK) cells for the treatment of hematological malignancies has revolutionized the fields of adoptive cell immunotherapy (5, 6). Despite the considerable effects of engineered technology using CAR loading in immunotherapy, both the cells are still inefficient because of their low potential against solid tumors. Human γδ T cells simultaneously possess properties of both T and NK cells (7). These properties, such as human leucocyte antigen (HLA)-independent antitumor activity; low risk for graft versus host disease (GvHD); multivalent response against a wide range of tumors, including solid ones; innate immune cells with memory function; and the presence of surface markers render γδ T cells as ideal universal allogeneic cells (3, 8, 9). γδ T cells mirror NK cells, expressing the NK receptor NKG2D and showing cytotoxicity against stressed and abnormal cells, such as viral-infected and tumor cells (10, 11). The 50∼95% PB-derived γδ T cells are Vδ2Vγ9 T cells expressing the NKG2D receptor (4). As human γδ T cells can recognize transformed cells through the NKG2D receptor, the activation and presence of the NKG2D receptor in Vδ2+ T cells are important to treat cancers (12, 13). These γδ T cells possessing properties of both T and NK cells in vivo can provide strong opportunity for neo-tumor associated antigens without genetic engineering. In clinical trials, PB-derived γδ T cells and ex vivo expanded γδ T cells are commonly used in cancer treatment. Vδ2Vγ9 T cells function as a strong anti-tumoral effector due to the abundant release of cytolytic effector molecules, such as perforin and granzyme B, if the limitation of low cell number is overcome. To overcome this limitation, an ex vivo expansion of Vδ2Vγ9 T cells using a cell culture cocktail containing IL-2, 4.1BB, CD80, CD83 ligands and zoledronate was developed (3, 14, 15). The clinical unmet needs for the use of PSC-derived γδ T cells are gradually increasing in immunotherapy.
Human pluripotent stem cells (hPSCs), including embryonic and induced PSCs, provide renewable sources of cells that can be genetically engineered and differentiated into immune lineage cells with functionality. Hemato-poietic cells, including myeloid and lymphoid lineage cells in early embryos, originate from the definitive hemogenic endothelium (HE). Functional hematopoiesis, including hematopoietic stem cells and lymphoid lineage cells, is generated from definitive HE (16). Timmermans et al. (17) showed that T cells expressing TCRγδ are generated from H1 ESCs, when OP9 stromal cells are co-cultured. Also, Chang et al. (18) has reported that broad TCR repertoires, including γδ and αβ of iPSC-derived T cells, emerge as the differentiation progresses. However, no studies have clearly addressed the induction of γδ T cells from PSC-derived HE.
In our study, we investigated whether PSCs can generate γδ T cells with pivotal transcriptional factors via HE (19, 20). PSCs continuously proliferate and can be differentiated into all lineage cells; therefore, PSCs are considered a potent cell source for universal allogeneic CAR-immune cells, including T, NK and γδ T cells, in immunotherapy.
These approaches that rely on mimicked aspects of developmental biology facilitated the development of γδ T cells from PSCs. Moreover, differentiated γδ T cells might provide a platform for drug screening and be directly used as immunotherapeutic cells in the clinic.
All experiments proceeded under authorization from the Institutional Review Board for Human Research at the CHA University (1044308-202204-LR-023-02). Undifferen-tiated human PSCs (CHA52 embryonic stem cell line) were maintained under vitronectin (07180, Stem Cell Tech-nologies Inc., Vancouver, BC, Canada) to avoid differentiation lineage cells with mTeSRTM 1 medium (85850; Stem Cell Technologies Inc., Vancouver, BC, Canada), and then seeded onto Matrigel®-coated plates (354234, corning) with APELTM 2 medium to differentiate HE from PSCs. The cells were incubated with APELTM 2 medium supplemented with 3 nM CHIR-99021 (S2924, Selleck Chemi-cals, Houston, TX, USA), 20 ng/ml of vascular endothelial growth factor (VEGF)165 (100-20; PeproTech Inc., Rocky Hill, NJ, USA), and 25 ng/ml HumanKine® BMP-4 (HZ-1045; Proteintech Group Inc., Rosemont, IL, USA) for three days to induce mesoderm. To induce the HE, cells were then incubated with APELTM 2 medium supplemented with 25 ng/ml HumanKine® BMP-4 (HZ-1045, Proteintech), and 250 ng/ml stem cell factor (SCF; 300-07), 20 ng/ml VEGF165 (100-20), 200 ng/ml FMS-like tyrosine kinase 3 (Flt3)-Ligand (300-19), 100 ng/ml Thro-mbopoietin (TPO) (300-18) and 20 ng/ml erythropoietin (EPO) (100-64; all from PeproTech) for two days. For further development, 80 ng/ml IL-5 (200-05), 5 ng/ml IL-7 (200-07), 60 ng/ml IL-15 (200-15), and 25 ng/ml DLL-1 (140-08; all from PeproTech) were added in culture medium according to plan till 39 days. The schematic plan was shown in Fig 1.
The PSCs, HE, and γδ T cells derived from PSCs were incubated with antibodies, washed with 0.5% FBS in PBS, and analyzed using a BD Accuri C6 plus flow cytometer (BD Biosciences, San Jose, CA, USA). The antibodies used in this study were as follows: FITC-conjugated mouse anti-human CD34 (348053, BD Biosciences), goat anti-human TIE-2 (AF313, R&D Systems Inc., Minneapolis, MN, USA), PE-conjugated mouse anti-human CD3 (300408), PE-conjugated mouse anti-human TCR Vγ9 (331308), and FITC-conjugated mouse anti-human TCR Vδ2 (331406; all from BioLegend, San Diego, CA, USA), SimultestTM FITC/PE-conjugated anti-human CD3/CD-16+CD56+ (340042; BD Biosciences, San Diego, CA, USA), APC-conjugated anti-human NKG2D (320808; BioLegend, San Diego, CA, USA) and APC-conjugated Mouse Anti-Human CD161 (550968; BD Pharmingen, San Diego, CA, USA), APC-conjugated mouse anti-human CD73 (344006, Biolegend, San Diego, CA, USA), PE-conjugated mouse anti-human CXCR4 (555974, BD Pharmingen, San Diego, CA, USA).
HE and γδ T cells were fixed with 2% PFA and permeabilized with 0.25% Triton X-100 for 20 min at room temperature. The cells were then washed with PBS, non-specific antibody binding was blocked with 5% normal goat serum, and the cells were incubated with primary, followed by secondary antibodies. Nuclei were stained with 4’, 6-diamidino-2-phenylindole (DAPI; Vector Laboratories Inc., Burlingame, CA, USA), and the cells were visualized using an IX73 fluorescent microscope (Olympus Optical Co., Ltd., Tokyo, Japan), or an Axio Imager.a2 (Carl Zeiss AG., Oberkochen, Germany). mouse anti-human CD34 (826401, BioLegend), goat anti-human TIE-2 (AF313, R&D Systems Inc.). FITC-conjugated Mouse Anti-Human CD56 (340410; BD Biosciences), APC-conjugated anti-human NKG2D (320808; BioLegend) were used as a primary antibodies. Primary signals were detected using isotype-matched IgG antibodies.
Lymphoid lineage cells derived from PSCs were dropped onto microscope slides and air-dried, then cells were stained with Wright-Giemsa Solution (ab245888, Abcam) as described by the manufacturer. Briefly, the cells were fixed in methanol for 5 minutes, and then stained with Wright-Giemsa solution for 5 min. The cells were washed with distilled water, rinsed with PBS, and covered with the mounting solution.
Total RNA was extracted from human cells using TRIzol reagent (Ambion, Austin, TX, USA) as described by the manufacturer. cDNA was then synthesized using reverse transcriptase kits (RT200, Enzynomics, Daejeon, South Korea). Fragments were amplified by qRT-PCR using primers (Table 1), SYBR Green (RT500M, Enzy-nomics), and the CFX96TM Real-Time System (Bio-Rad La-boratories Inc., Hercules, CA, USA). The relative mRNA expression of target genes was calculated using the comparative CT method. All target genes were normalized to 18s in multiplexed triplicate reactions. Differences in CT values were calculated for each target mRNA by subtracting the mean value of 18s (relative expression=2‐ΔCT).
We developed a protocol in which γδ T cells from hPSCs were cultured for 39 days in a medium supplemented with specific cytokines. Three days after differentiation, the cells reached the mesodermal stage and HE was detected in the cultured cells at day 6. To generate lymphoid lineage γδ T cells, differentiated cells were further incubated under defined culture conditions supplemented with IL-6, -7, and -15 series. Previously, we had found that the APELTM 2 medium was appropriate to generate blood lineage cells and for endothelial-to-hematopoietic transition (EHT) with cytokine supplementation. HE in the aorta-gonad-mesonephros vigorously generated erythroblasts and myeloid lineage cells, and these blood lineage cells were grown in fetal liver after infiltration with HE. The protocol designed was analogous to the in vivo developmental stage for blood lineage cell generation (Fig. 1). As lymphoid lineage cells, including γδ T, CD3, and NK cells, are proliferated and maturated by niche factors in the liver, we added critical cytokines for lymphoid lineage commitment.
Using the protocol mentioned above, we investigated whether definitive HE was successfully generated from PSCs, and what percentage of HE was present under that culture condition. At day 3 after differentiation, mesodermal lineage markers were detected in differentiated cells (data not shown). The microscopic images showed that HE cell populations were clearly detected in the plate at days 5 and 6. (Fig. 2A) In addition, pan HE marker TIE-2 and CD34 cells were unarguably expressed in adherent differentiated cells at day 6, suggesting the presence of HE under defined culture conditions (Fig. 2B). To quantify HE cells, flow cytometry was performed using differentiated cells at days 5 and 6. As shown in Fig. 2B, TIE-2 and CD34 markers for conventional HE at day 5 were expressed in HE at 27.1±2.2% and 30.4±2.7%, respectively. These markers were also notable in HE cells on day 6 (TIE-2, 33.4±8.2%; CD34, 30.1±7.4%), suggesting that differentiated cells continued producing HE at that time (Fig. 2C). To address definitive HE, the percentage of CXCR4-CD73- cells in Tie-2+ and CD34+ cells was calculated by FACS analysis. Results showed that CXCR4-CD73- cells in TIE-2+ and CD34+ cells at day 5 were expressed in HE at 9.5±2.3% and 82.7±4.4%, respectively. These markers were also displayed in HE on day 6 (TIE-2, 2.1±0.1%; CD34, 64.6±1.0%), suggesting the superior role of CD34 in definitive HE (Fig. 2D). Thus, we putatively considered that these HE functioned as definitive HE and confirmed that the lymphoid lineage γδ T cells were successfully generated via HE under defined culture conditions.
We further cultured cells for 39 days using HE to examine whether lymphoid lineage γδ T cells were generated from PSCs under defined culture conditions. EHT occurred on the HE and the numbers of budding erythroblasts considerably increased between 10 and 21 days of HE incubation, suggesting bona fide HE production of blood lineage cells (Fig. 3A). From day 21, clustered lymphoblasts were detected in floating cells. Blood cells, including single cells and cell clusters, were collected until day 31 and transferred onto a new plate without HE. The suspended cells, which were transferred onto a new culture plate, created a microenvironment and were continuously generated for 39 days on newly adherent cells. Wright-giemsa staining results showed that lymphoid lineage committed cells displayed very rounded and condensate cytoplasm without granules (Fig. 3A, lower right panel). To quantify the numbers of γδ T cells in floating cells, flow cytometry was conducted using differentiated cells at days 26 and 30. The percentages of CD3, CD161+CD3+, and NKG2D+CD3+ cells were maximal at 7.1±3.1%, 9.5± 1.3%, and 46.4±6.7%, respectively, on day 26. Meanwhile, higher percentages for γδ T cells were determined in the differentiated cells at day 30 (CD3, 21.5±5.4%; CD161+ CD3+, 91.7±1.7%; and NKG2D+CD3+, 84.1±3.4%; Fig. 3B), suggesting the successful generation of γδ T cells. In addition, the immunocytochemistry results showed that the markers CD56 and NKG2D for γδ T cells were expressed in differentiated cells (Fig. 3C). When generating PSC-derived differentiated cells, γδ T cells were detected in two types of cells; HE and floating cells. Similarly with previous reports, (21) γδ T cells in our culture condition were detected in HE as well as floating cells. Thus, we tested the numbers of γδ T cells in both HE and floating cells at day 26. Representative markers for γδ T, Vδ2+CD3+, and Vγ9+CD3+ cells had no significant differences in their numbers (Vδ2+CD3+ cells; HE, 10.3± 5.9%, floating cells, 25.0±11.0%, Vγ9+CD3+ cells; HE, 11.0±5.6%, floating cells, 11.8±6.2%; Fig. 3D). To test γδ T cells after complete differentiation, we examined the percentages of CD3, Vδ2, Vγ9, and Vδ2+Vγ9+ in CD3+ cells at day 31 using HE and floating cells. T cell receptor (TCR) Vδ2+ cell number significantly increased at day 31 compared to that of day 21 in floating cells, suggesting the optimization of the culture conditions for the generation of γδ T cells. (CD3; HE, 8.5±2.9%, floating cells, 14.2±5.1%, Vδ2+ cells; HE, 14.8±2.0%, floating cells, 45.5±5.2%, Vγ9+ cells; HE, 11.4±1.1%, floating cells, 25.4±5.5%, Vδ2+ Vγ9+ in CD3 cells; HE, 74.5±5.8%, floating cells, 86.0±7.9%; Fig. 3E). Next, to investigate whether transcription factors involving γδ T cells increased under defined conditions with generation of γδ T cells, real-time PCR was performed using differentiated cells as well as PSCs and HE. The expressions of OCT4 and Nanog genes gradually decreased and the expression of KDR also deceased after a peak at day 6. Markers for HE, TIE-2, and CD34 had similar expression patterns as KDR. Meanwhile, the expression of RUNX1 and CD8 markers for hematopoietic lineage commitments increased at days 25 and 30 (CD8, 3.1-fold compared to that at day 6). The expression of lymphoid lineage markers, including t-BET and EOMES, gradually increased at day 30, showing lymphoid lineage commitments and the possibility of definitive hematopoiesis. Perforin and granzyme B transcript levels highly increased at day 30 (perforin, 3.7-fold, granzyme B, 5.2-fold compared to those at day 6). All PCR results showed the proper conditions for differentiation into γδ T cells.
γδ T cells are promising cell sources for immunologists because of their unique developmental paradigm and functional benefits, which can be exploited for immuno-therapy. Among the TCR variants, Vδ2 in γδ T cells pairs with a TCR composed of the variable gene Vγ9. Vγ9Vδ2 γδ T cells, which dominantly prevail in the PB cells sharing the characteristics of NK and T cells simultaneously, are activated by endogenous tumors and microbes by two independent mechanisms, including cytokine production and cytotoxicity (22). Especially, Vδ2 γδ T cells have cytotoxic effector functions via binding NKG2D/NKG2D ligand ULBP series and MICA/B interactions regardless of TCR signaling, suggesting NK properties against transformed tumors (10, 23); whereas, γδ T cells activating TCR are classified as T lymphocytes with memory function as an adaptive immune responder. Thus, γδ T cells are potent effector cells that can be used in the clinic, suggesting their function as adaptive immune cells (24). γδ T cells possess beneficial properties of both T and NK cells. Since CAR-T and CAR-NK cells are used in patients with cancer (5, 6), many trials have reported the generation of tumor-targeted human lymphocytes from PSCs with diverse CAR loading (25-27). However, the major drawback of CAR-T production was the limitation that the TCR repertoire rearrangement is unpredictable and only allows loaded target molecules because the TCR rearranges randomly upon differentiation of PSC-derived CAR-T or NK cells in vitro. Thus, some studies report that PSC-derived, CAR-expressing T cells display a phenotype resembling that of innate γδ T cells (25). γδ T cells are progenitors for immature T cells, assembling the TCR rearrangement by education of HLA class in γδ T cells. This might be the reason why injected CAR-T cells function as γδ T cells due to the loss of diverse HLA series, when most CAR-T cells were derived from induced PSCs. Although Vγ9Vδ2 γδ T cells have a primitive character, regardless of CAR addition, they dual function to directly enhance antitumoral effects and indirectly exert CD4 and 8 cells against tumors as antigen presenting cells. Due to the potency of γδ T cells against tumors, the anticancer effect of these cells is considered important as T cells engineered with Vγ9Vδ2 TCR are manufactured in a good manufacturing practice (GMP) facility (28). In addition, the combination of NK cells with Vγ9Vδ2 TCR might reveal their synergistic potential in cancer treatment. As mentioned above, the safety of allogenic γδ T cells in graft-versus-host disease (GvHD) occurrence and normal brain damage was confirmed. Despite many advantages, adult PB-derived γδ T cells have drawbacks to be used in the clinics. To solve this problem, the approach of generating PSC-derived lymphoid lineage cells is emerging in the field of cancer biology. The technical advancement in the differentiation of PSCs answers many unsolved questions in regenerative medicine and cancer biology. All blood lineage cells are generated from HE, which is an established pivotal reservoir having bipotent to generate endothelial and hematopoietic lineage cells. Using definitive HE cells, we developed a protocol for differentiating γδ T cells under the following conditions: non-embryonic bodies, fewer cytokines, serum-free media, and non-stromal conditions. These PSC-derived γδ T cells may provide an opportunity to deliver allogeneic T cell therapies without GVHD, suggesting true “off-the-shelf” synthetic γδ T cells. Because the γδ T cells that are generated from PSCs in present study maintained the high frequency without OP9 stromal cells, it favorable to apply industry, compared to that shown in previous papers (17, 18). Also, we will next consider ex vivo expanded γδ T cells to purify γδ T cells by zoledronate and interleukin-2 (14). The clinical applications of PSC-derived lineage cells require at least system reproducibility, simplicity, cost-effectiveness, and being xenogeneic-free. In the present study, we established the protocol for the generation of γδ T cells based on the above-mentioned description.
This differentiation method for γδ T cells with specifications from our protocol might serve as an early in vitro platform to study cell-based therapy, especially hematopoietic lymphoid lineage cells, as well as mechanistic studies in the field of cancer biology.
References
1. Saito H, Kranz DM, Takagaki Y, Hayday AC, Eisen HN, Tonegawa S. 1984; Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. Nature. 309:757–762. DOI: 10.1038/309757a0. PMID: 6330561.
2. Gomes AQ, Martins DS, Silva-Santos B. 2010; Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 70:10024–10027. DOI: 10.1158/0008-5472.CAN-10-3236. PMID: 21159627.
3. Alnaggar M, Xu Y, Li J, He J, Chen J, Li M, Wu Q, Lin L, Liang Y, Wang X, Li J, Hu Y, Chen Y, Xu K, Wu Y, Yin Z. 2019; Allogenic Vγ9Vδ2 T cell as new potential immu-notherapy drug for solid tumor: a case study for cholangio-carcinoma. J Immunother Cancer. 7:36. DOI: 10.1186/s40425-019-0501-8. PMID: 30736852. PMCID: PMC6368763.
4. Yazdanifar M, Barbarito G, Bertaina A, Airoldi I. 2020; γδ T cells: the ideal tool for cancer immunotherapy. Cells. 9:1305. DOI: 10.3390/cells9051305. PMID: 32456316. PMCID: PMC7290982. PMID: 28a5b0d092124d3391cbaffa2b72afbd.
5. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY. 2017; Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 377:2531–2544. DOI: 10.1056/NEJMoa1707447. PMID: 29226797. PMCID: PMC5882485.
6. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M, Nandivada V, Kaur I, Nunez Cortes A, Cao K, Daher M, Hosing C, Cohen EN, Kebriaei P, Mehta R, Neelapu S, Nieto Y, Wang M, Wierda W, Keating M, Champlin R, Shpall EJ, Rezvani K. 2020; Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 382:545–553. DOI: 10.1056/NEJMoa1910607. PMID: 32023374. PMCID: PMC7101242.
7. Lanier LL. 2013; Shades of grey--the blurring view of innate and adaptive immunity. Nat Rev Immunol. 13:73–74. DOI: 10.1038/nri3389. PMID: 23469373.
8. Li R, Johnson R, Yu G, McKenna DH, Hubel A. 2019; Preser-vation of cell-based immunotherapies for clinical trials. Cyto-therapy. 21:943–957. DOI: 10.1016/j.jcyt.2019.07.004. PMID: 31416704. PMCID: PMC6746578.
9. Vantourout P, Hayday A. 2013; Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol. 13:88–100. DOI: 10.1038/nri3384. PMID: 23348415. PMCID: PMC3951794.
10. Rincon-Orozco B, Kunzmann V, Wrobel P, Kabelitz D, Steinle A, Herrmann T. 2005; Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol. 175:2144–2151. DOI: 10.4049/jimmunol.175.4.2144. PMID: 16081780.
11. Wiemann K, Mittrücker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. 2005; Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J Immunol. 175:720–729. DOI: 10.4049/jimmunol.175.2.720. PMID: 16002667.
12. Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. 1999; Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 285:727–729. DOI: 10.1126/science.285.5428.727. PMID: 10426993.
13. Kong Y, Cao W, Xi X, Ma C, Cui L, He W. 2009; The NKG2D ligand ULBP4 binds to TCRgamma9/delta2 and induces cytotoxicity to tumor cells through both TCRgammadelta and NKG2D. Blood. 114:310–317. DOI: 10.1182/blood-2008-12-196287. PMID: 19436053.
14. Cho HW, Kim SY, Sohn DH, Lee MJ, Park MY, Sohn HJ, Cho HI, Kim TG. 2016; Triple costimulation via CD80, 4-1BB, and CD83 ligand elicits the long-term growth of Vγ9Vδ2 T cells in low levels of IL-2. J Leukoc Biol. 99:521–529. DOI: 10.1189/jlb.1HI0814-409RR. PMID: 26561569.
15. Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D'Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. 2007; Targeting human γδ T cells with zoledronate and interleukin-2 for immunothe-rapy of hormone-refractory prostate cancer. Cancer Res. 67:7450–7457. DOI: 10.1158/0008-5472.CAN-07-0199. PMID: 17671215. PMCID: PMC3915341.
16. Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML. 2008; Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 3:625–636. DOI: 10.1016/j.stem.2008.09.018. PMID: 19041779. PMCID: PMC2631552.
17. Timmermans F, Velghe I, Vanwalleghem L, De Smedt M, Van Coppernolle S, Taghon T, Moore HD, Leclercq G, Langerak AW, Kerre T, Plum J, Vandekerckhove B. 2009; Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J Immunol. 182:6879–6888. DOI: 10.4049/jimmunol.0803670. PMID: 19454684.
18. Chang CW, Lai YS, Lamb LS Jr, Townes TM. 2014; Broad T-cell receptor repertoire in T-lymphocytes derived from human induced pluripotent stem cells. PLoS One. 9:e97335. DOI: 10.1371/journal.pone.0097335. PMID: 24828440. PMCID: PMC4020825.
19. Zhong C, Zhu J. 2017; Transcriptional regulators dictate innate lymphoid cell fates. Protein Cell. 8:242–254. DOI: 10.1007/s13238-017-0369-7. PMID: 28108952. PMCID: PMC5359184.
20. Laurenti E, Doulatov S, Zandi S, Plumb I, Chen J, April C, Fan JB, Dick JE. 2013; The transcriptional architecture of early human hematopoiesis identifies multilevel control of lymphoid commitment. Nat Immunol. 14:756–763. DOI: 10.1038/ni.2615. PMID: 23708252. PMCID: PMC4961471.
21. Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, Deban L, Cipolat S, Hart R, Iannitto ML, Laing A, Spencer-Dene B, East P, Gibbons D, Irving PM, Pereira P, Steinhoff U, Hayday A. 2016; Epithelia use butyrophilin-like molecules to shape organ-specific γδ T cell compartments. Cell. 167:203–218.e17. DOI: 10.1016/j.cell.2016.08.030. PMID: 27641500. PMCID: PMC5037318.
22. Cano CE, Pasero C, De Gassart A, Kerneur C, Gabriac M, Fullana M, Granarolo E, Hoet R, Scotet E, Rafia C, Herrmann T, Imbert C, Gorvel L, Vey N, Briantais A, le Floch AC, Olive D. 2021; BTN2A1, an immune checkpoint targeting Vγ9Vδ2 T cell cytotoxicity against malignant cells. Cell Rep. 36:109359. DOI: 10.1016/j.celrep.2021.109359. PMID: 34260935.
23. Paczulla AM, Rothfelder K, Raffel S, Konantz M, Steinbacher J, Wang H, Tandler C, Mbarga M, Schaefer T, Falcone M, Nievergall E, Dörfel D, Hanns P, Passweg JR, Lutz C, Schwaller J, Zeiser R, Blazar BR, Caligiuri MA, Dirnhofer S, Lundberg P, Kanz L, Quintanilla-Martinez L, Steinle A, Trumpp A, Salih HR, Lengerke C. 2019; Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature. 572:254–259. Erratum in: Nature 2019;572:E19. DOI: 10.1038/s41586-019-1410-1. PMID: 31316209. PMCID: PMC6934414.
24. Silva-Santos B, Serre K, Norell H. 2015; γδ T cells in cancer. Nat Rev Immunol. 15:683–691. DOI: 10.1038/nri3904. PMID: 26449179.
25. Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, Sadelain M. 2013; Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 31:928–933. DOI: 10.1038/nbt.2678. PMID: 23934177. PMCID: PMC5722218.
26. Li Y, Hermanson DL, Moriarity BS, Kaufman DS. 2018; Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 23:181–192.e5. DOI: 10.1016/j.stem.2018.06.002. PMID: 30082067. PMCID: PMC6084450.
27. Jing R, Scarfo I, Najia MA, Lummertz da Rocha E, Han A, Sanborn M, Bingham T, Kubaczka C, Jha DK, Falchetti M, Schlaeger TM, North TE, Maus MV, Daley GQ. 2022; EZH1 repression generates mature iPSC-derived CAR T cells with enhanced antitumor activity. Cell Stem Cell. 29:1181–1196.e6. DOI: 10.1016/j.stem.2022.06.014. PMID: 35931029.
28. Straetemans T, Kierkels GJJ, Doorn R, Jansen K, Heijhuurs S, Dos Santos JM, van Muyden ADD, Vie H, Clemenceau B, Raymakers R, de Witte M, Sebestyén Z, Kuball J. 2018; GMP-grade manufacturing of T cells engineered to express a defined γδTCR. Front Immunol. 9:1062. DOI: 10.3389/fimmu.2018.01062. PMID: 29899740. PMCID: PMC5988845.
Fig. 1
Schematic for generating γδ T cells from PSCs via HE according to developmental biology. There are four steps to differentiate into γδ T cells: stage I, yolk sac blood island to mesodermal lineage; stage II, HE in aorta-gonad mesonephros; stage III, pre γδ T cells development in fetal liver; stage IV, generation of γδ T cells and growth in fetal liver. Diverse cytokines were added in each step to optimize the generation of γδ T cells.
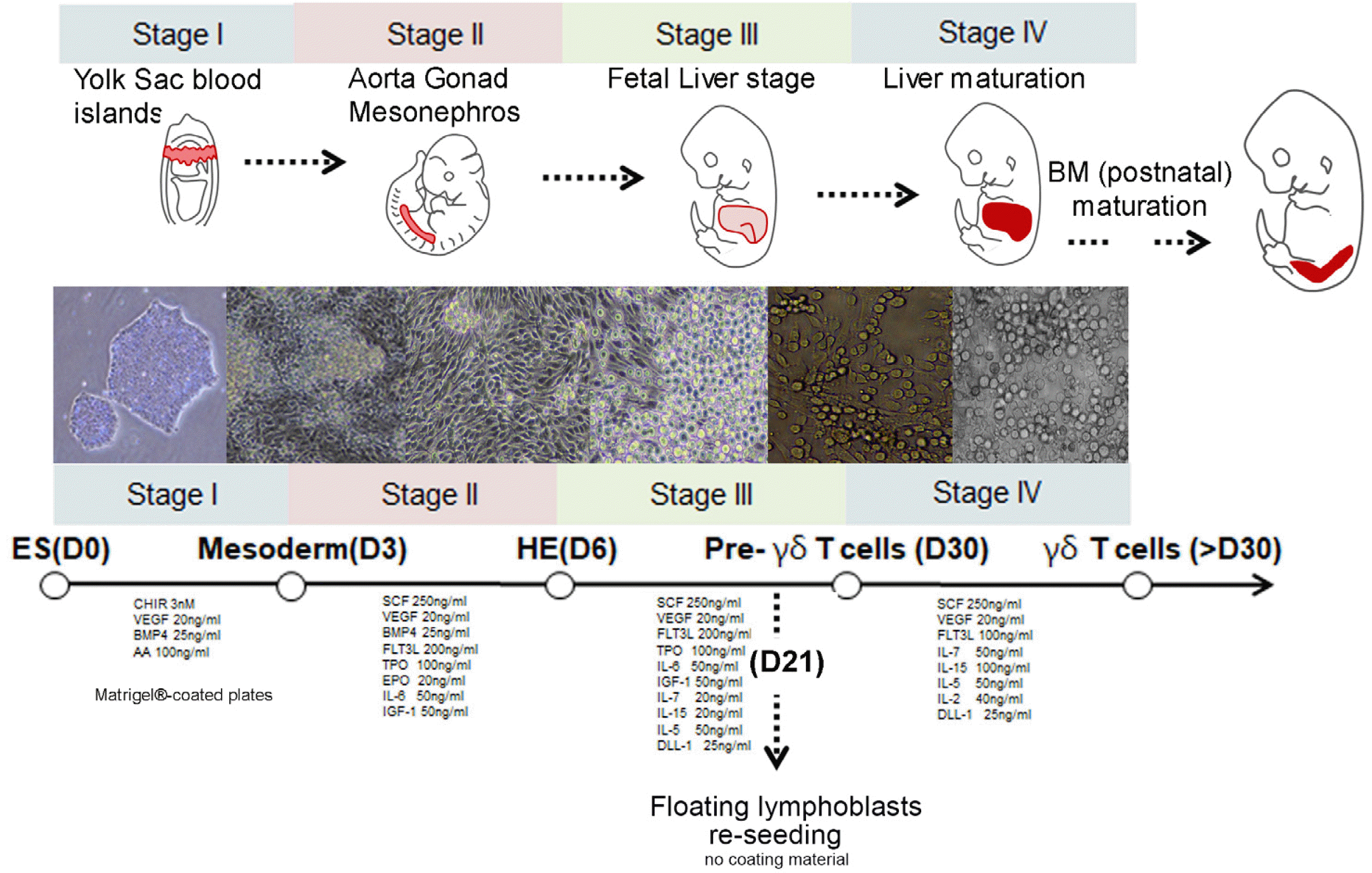
Fig. 2
HE from PSCs was stably generated at days 5 and 6. (A) Representative morphology of PSC-derived HE according to BMP4. At days 5 and 6, yellow lines depict HE territory. Magnification 20×. (B) At day 6, the TIE-2 and CD34 marker for HE was expressed in adherent cells. TIE-2 (Green), CD34 (red), and DAPI (blue) for nuclear staining are demarcated in HE. Magnification 20× and 40×. (C) Percentage of TIE-2 and CD34 cells in differentiated cells at days 5 and 6. Images are representative of at least two independent experiments. (D) The percentage of CXCR4-CD73-cells in TIE-2+ cells and CD34+ cells in differentiated cells at days 5 and 6. Images from three independent experiments are shown.
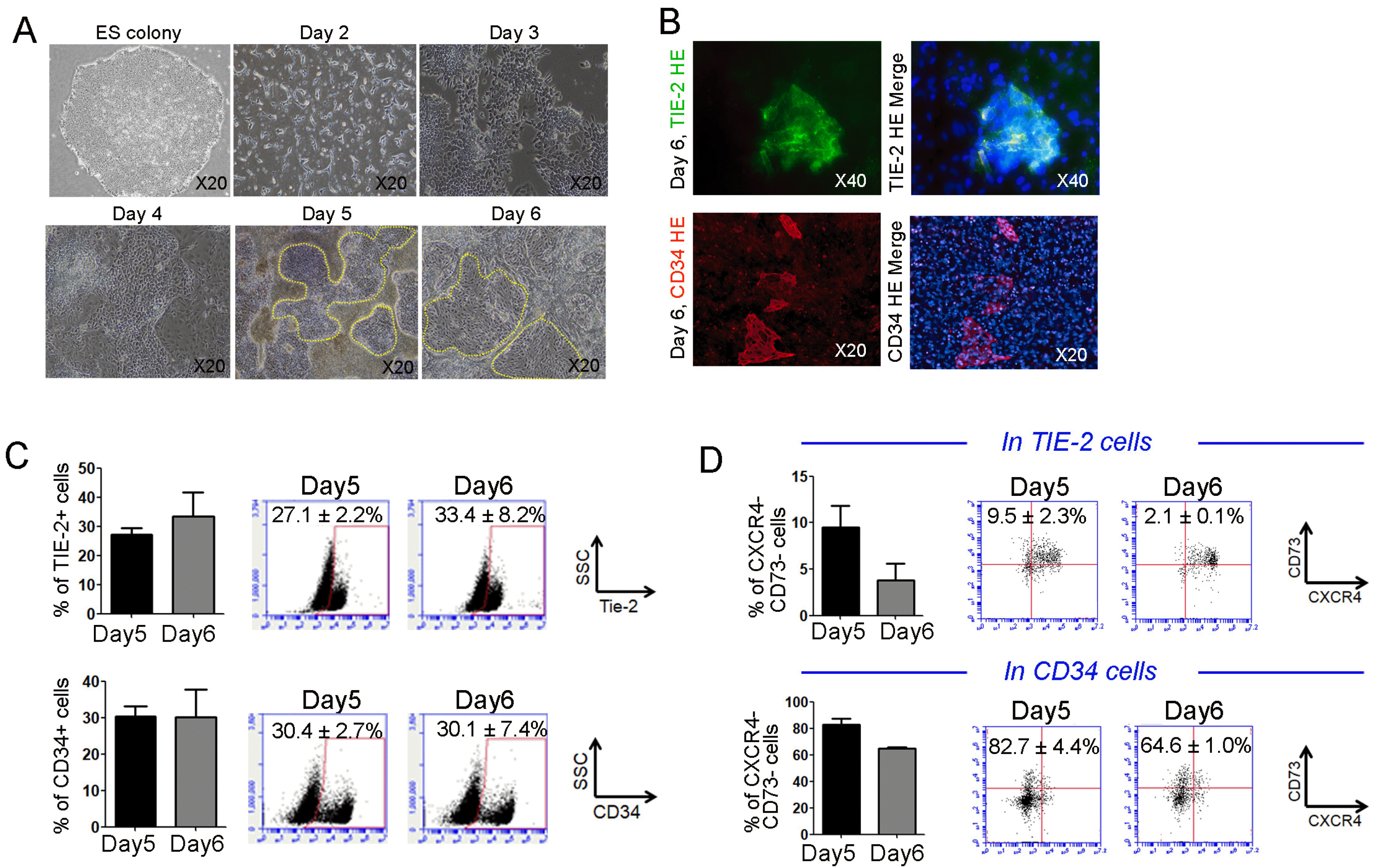
Fig. 3
γδ T cells from HE were successfully generated under defined culture conditions. (A) Representative morphology of γδ T cells from HE until day 39. Wright and Giemsa staining showed lymphoid lineage cells. Magnification 20×. (B) Flow cytometry results revealed that markers for γδ T cells were expressed in differentiated cells at days 26 and 30. The numbers of γδ T cells were significantly increased. Results are shown as the means±SEM from at least two independent experiments *p<0.05, **p<0.01. (C) Immunochemistry findings for CD56 and NKG2D expression in differentiated cells, including HE and floating cells, on day 26. Magnification 20×. (D) FACS results showed no difference in the percentages of Vδ2+CD3+ and Vγ9+CD3+ cells in HE and floating cells, respectively. Results are from at least three independent experiments. (E) FACS results revealed that the numbers of CD3, TCR Vδ2+, TCR Vγ9+, and Vδ2+Vγ9+ in CD3 cells gradually increased over time in the floating cells than those in HE. Results are shown as the means±SEM from at least two independent experiments. *p<0.05. (F) Genes according to each developing steps were determined in differentiated cells. mRNA expression of representative marker genes was determined through qRT-PCR. Results are shown as the means±SEM.
Table 1
Primers for quantitative RT-PCR




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download