Candidemia is a major cause of hospital-acquired infections and mortality [
1-
3]. The
Candida cell wall polysaccharide (1–3)-β-D-glucan (BDG) is widely used as an antigenic marker for early diagnosis of invasive candidiasis, including candidemia [
4,
5]. The BDG test is used to decide on the use of antifungal agents and discontinuation of empirical antifungal therapy in patients at risk of invasive candidiasis [
6,
7]. However, the performance of the test varies substantially [
4,
5,
8]. We analyzed the sensitivity of the Goldstream Fungus (1–3)-β-D-Glucan Test for predicting candidemia and evaluated the clinical and microbiological characteristics of patients with positive BDG results before or on the day of candidemia onset.
This retrospective study included 93 adult patients with candidemia who underwent a serum BDG test within seven days prior to the onset of candidemia at Asan Medical Center—a 2,700-bed tertiary referral hospital in Seoul, Korea—between January 2017 and May 2021. Patients were identified by cross-checking candidemia cases in a database of BDG test results. Data on age, sex, admission department, underlying disease, risk factors for candidemia, clinical presentation, origin of candidemia, Candida species, antifungal therapy, serum BDG, and outcome were collected, and the clinical characteristics and outcomes of patients with any-positive BDG results versus all-negative BDG results were compared. The Institutional Review Board of Asan Medical Center approved this study (approval number 2022-0873). The requirement for obtaining informed consent from the patients was waived given the observational and retrospective nature of the study.
Candidemia was defined as the isolation of
Candida species from blood in patients with signs and symptoms of infection. Candidemia onset was defined as the date of the first culture-positive blood sample. Any-positive BDG results were defined as at least one positive result in the performed BDG tests. Other definitions are provided in the
Supplemental Data.
Blood was cultured in Bactec Plus Aerobic/F and Bactec Lytic/10 Anaerobic/F vials (Becton Dickinson DIS, Sparks, MD, USA), according to the manufacturer’s instructions. Yeasts were identified using a Vitek 2 YST card (bioMérieux, Marcy l’Étoile, France) and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker Daltonik, Bremen, Germany). The blood samples were subjected to the Goldstream Fungus (1–3)-β-D-Glucan Test (GKT-12M; Gold Mountain River Tech Development, Beijing, China). Values above the maximum detectable level (1,000 pg/mL) were recorded as >1,000 pg/mL. The cut-off value for a positive BDG result was 80.0 pg/mL, according to the manufacturer’s instructions.
Categorical variables were analyzed using Pearson’s chi-square test or Fisher’s exact test, and continuous variables using the Mann–Whitney U-test. P<0.05 was considered to indicate significance. Data were managed and analyzed using IBM SPSS Statistics for Windows version 21.0 (IBM, Armonk, NY, USA) or R version 4.0.4 (R Project for Statistical Computing, Vienna, Austria).
During the study period, 576 adults were diagnosed as having candidemia, 93 of whom underwent a serum BDG test within seven days prior to the onset of candidemia, yielding 149 BDG serum samples in total (median, 1.6 tests per patient). The median age of the 93 patients was 63 years (range, 24-87 years), and 64.5% (60/93) were males. In total, 38 (40.9%) patients gave any-positive BDG results. The median BDG value in the any-positive BDG group was 254.9 pg/mL (interquartile range [IQR], 146.6-792.8 pg/mL).
Fig. 1 shows the proportions of positive BDG results at 0, 1, 2, 3-5, and 6-7 days before candidemia onset. The positive rate increased as the day of candidemia onset was approached (
P=0.04) but still reached only 52% (38% at 0-2 days before candidemia). Of the 93 patients, 37 (39.8%) underwent a BDG test in the first two days after candidemia, 21 of whom gave any-positive results.
Fig. 1
Proportions and distributions of the BDG results. (A) Proportions of positive BDG results at different time points before candidemia onset. The proportion of positive BDG results increased over time (P=0.04 for trend). (B) Distribution of the BDG values. Values above the maximum detectable level (1,000 pg/mL) were recorded as >1,000 pg/mL. The cut-off value for a positive BDG test was 80.0 pg/mL.
Abbreviation: BDG, (1–3)-β-D-glucan.
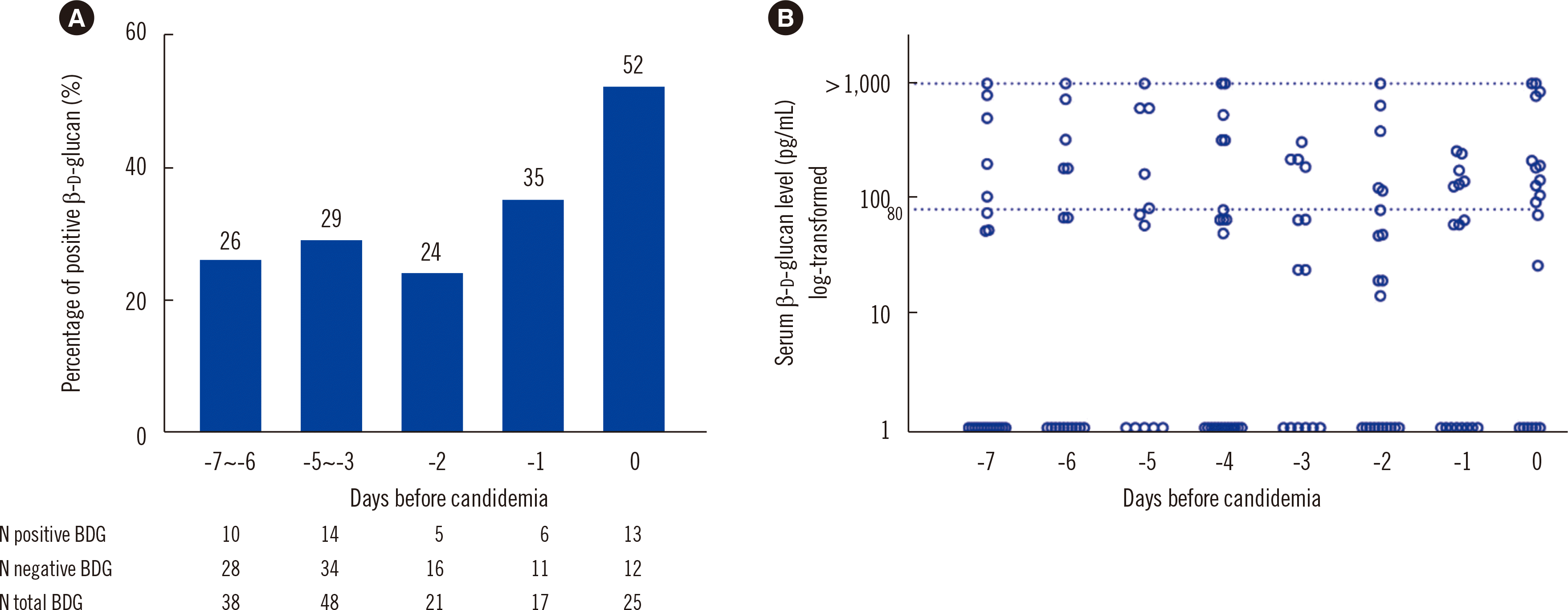

Table 1 compares the clinical and microbiological characteristics of the any-positive and all-negative BDG groups. There were no significant differences in terms of admission department, underlying disease, risk factors for candidemia, and clinical presentation. In the all-negative group, 17 (30.9%) patients received systemic antifungals within a month prior to candidemia onset, compared to 17 (44.7%) in the any-positive group (
P=0.17). The
Candida species detected are listed in
Table 1.
Candida albicans was significantly associated with any-positive BDG results than with all-negative BDG results (42.1% vs. 21.8%,
P=0.04).
Fig. 2 shows the distribution of BDG values according to
Candida species. The highest median value for positive BDG results was observed for
Candida tropicalis (median, 846 pg/mL; IQR, 231.9-1,000 pg/mL), followed by
C. albicans (median, 328.2 pg/mL; IQR, 158.9–816.4 pg/mL). There were no significant differences in the BDG values among the different
Candida species.
Fig. 2
Distribution of BDG values according to Candida species. Values above the maximum detectable level (1,000 pg/mL) were recorded as >1,000 pg/mL. The cut-off value for a positive BDG test was 80.0 pg/mL. Interspecies differences in BDG values were not statistically significant. The horizontal lines denote the median of positive BDG results.
Abbreviation: BDG, (1–3)-β-D-glucan.
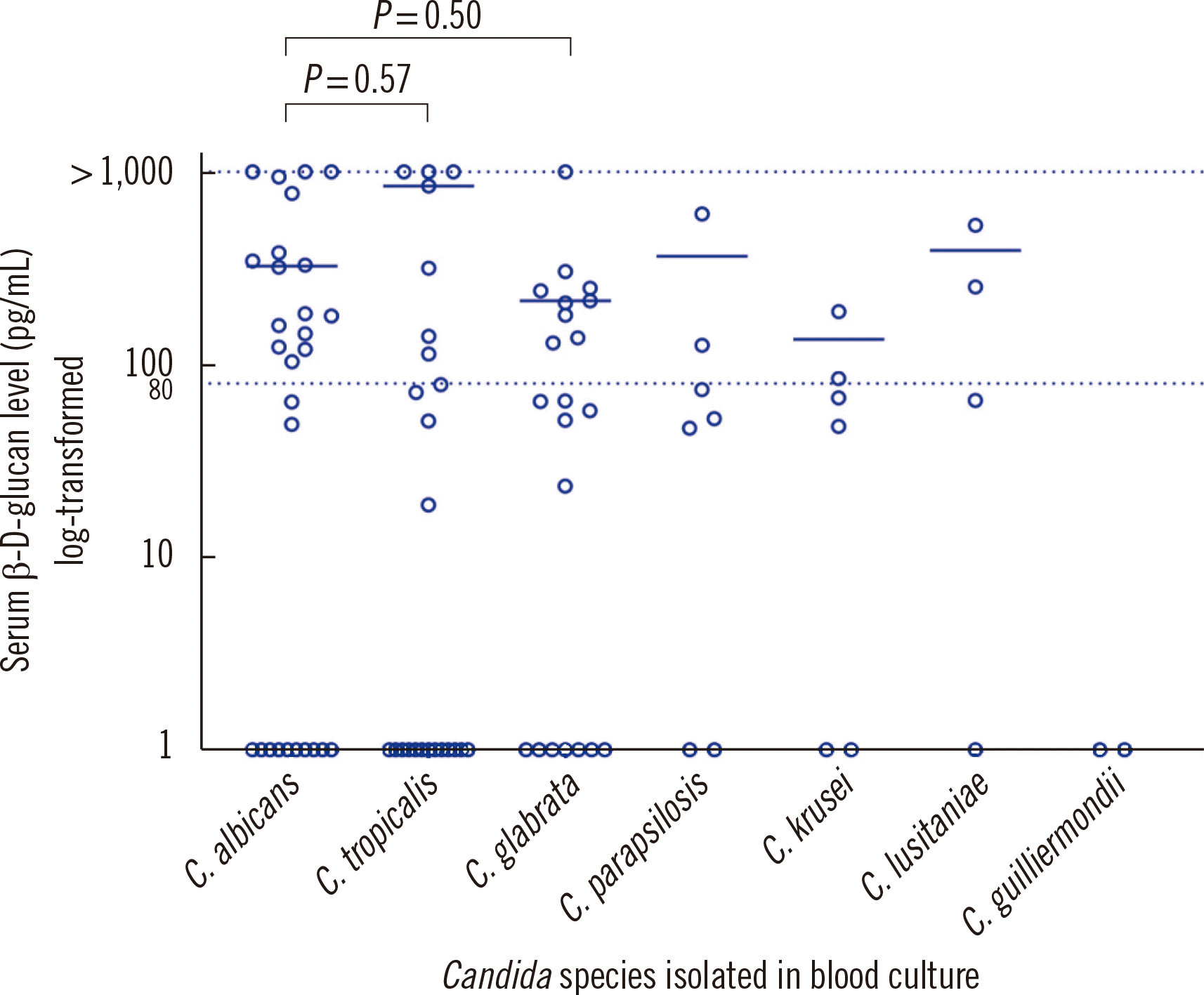

Table 1
Demographic and microbiological characteristics and outcomes of patients with candidemia according to positive vs. negative test results within seven days prior to candidemia onset
|
Characteristic |
Any-positive BDG results (N=38) |
All-negative BDG results (N=55) |
P
|
|
BDG value, median (IQR) |
254.9 (146.6-792.8) |
0 (0-49.3) |
|
|
Male |
22 (57.9) |
38 (69.1) |
0.28 |
|
Age, yr, median (IQR) |
62.5 (56.5-69.3) |
64 (53-72) |
0.26 |
|
Admission department |
|
|
|
|
Medical ward |
20 (52.6) |
24 (43.6) |
0.39 |
|
Surgical ward |
2 (5.3) |
3 (5.5) |
0.97 |
|
Medical ICU |
13 (34.2) |
21 (38.2) |
0.70 |
|
Surgical ICU |
3 (7.9) |
5 (9.1) |
0.84 |
|
Emergency room |
0 |
2 (3.6) |
0.24 |
|
Underlying disease*
|
|
|
|
|
Cardiovascular disease |
18 (47.4) |
22 (40.0) |
0.62 |
|
Hematologic malignancy |
16 (42.1) |
25 (45.5) |
0.75 |
|
Solid cancer |
10 (26.3) |
19 (34.5) |
0.54 |
|
Diabetes mellitus |
9 (23.7) |
18 (32.7) |
0.48 |
|
Bone marrow transplant |
7 (18.4) |
3 (5.5) |
0.10 |
|
Chronic liver disease |
6 (15.8) |
10 (18.2) |
0.98 |
|
Solid organ transplant |
6 (15.8) |
10 (18.2) |
0.98 |
|
Chronic kidney disease |
5 (13.2) |
4 (7.3) |
0.56 |
|
Risk factors for candidemia |
|
|
|
|
Previous antibiotics†
|
38 (100) |
52 (94.5) |
0.14 |
|
Total parenteral nutrition |
30 (78.9) |
40 (72.7) |
0.49 |
|
Previous antifungals†
|
17 (44.7) |
17 (30.9) |
0.17 |
|
Chemotherapy†
|
13 (34.2) |
24 (43.6) |
0.36 |
|
Candida colonization†
|
12 (31.6) |
21 (38.2) |
0.51 |
|
Antifungal use on the day of BDG test |
11 (28.9) |
15 (27.3) |
0.86 |
|
Neutropenia |
10 (26.3) |
20 (36.4) |
0.31 |
|
Surgery†
|
6 (15.8) |
3 (5.5) |
0.10 |
|
Clinical presentation |
|
|
|
|
Septic shock |
16 (42.1) |
23 (41.8) |
0.98 |
|
Pitt score, median (IQR) |
4 (0-7) |
4 (1-6) |
0.63 |
|
Pitt score ≥ 4 |
21 (55.3) |
30 (54.5) |
0.95 |
|
Origin of candidemia |
|
|
|
|
CVC-related |
22 (57.9) |
29 (52.7) |
0.78 |
|
Primary |
12 (31.6) |
18 (32.7) |
> 0.99 |
|
Intraabdominal |
2 (5.3) |
5 (9.1) |
0.77 |
|
Urinary tract |
1 (2.6) |
2 (3.6) |
> 0.99 |
|
Other‡
|
1 (2.6) |
1 (1.8) |
> 0.99 |
|
Candida species |
|
|
|
|
Candida albicans
|
16 (42.1) |
12 (21.8) |
0.04 |
|
Candida glabrata
|
9 (23.7) |
12 (21.8) |
0.83 |
|
Candida tropicalis
|
7 (18.4) |
18 (32.7) |
0.13 |
|
Candida parapsilosis
|
2 (5.3) |
5 (9.1) |
0.49 |
|
Candida krusei
|
2 (5.3) |
4 (7.3) |
0.70 |
|
Candida lusitaniae
|
2 (5.3) |
2 (3.6) |
0.70 |
|
Candida guilliermondii
|
0 |
2 (3.6) |
0.24 |
|
Antifungal therapy |
|
|
|
|
Echinocandin |
29/38 (76.3) |
45/53 (84.9) |
0.52 |
|
Liposomal amphotericin B |
5/38 (13.2) |
5/53 (9.4) |
0.53 |
|
Azole |
4/38 (10.5) |
3/53 (5.7) |
0.36 |
|
Time to antifungal therapy, days, median (IQR) |
1 (0-2) |
1 (1-2) |
0.38 |
|
Infection source control |
24/26 (92.3) |
25/34 (73.5) |
0.06 |
|
Time to source control from initial candidemia date, days, median (IQR) |
2 (1-4.75) |
2 (1-4) |
0.69 |
|
Persistent candidemia ( ≥ 5 days) |
2/38 (5.3) |
10/51 (19.6) |
0.05 |
|
30-day mortality |
23 (60.5) |
26 (47.3) |
0.21 |

We found no difference in the use of antifungal therapy, including echinocandins, between the groups (29/38 [76.3%] vs. 45/53 [84.9%],
P=0.52;
Table 1), but patients with any-positive BDG results may have had more adequate source control (24/26 [92.3%] vs. 25/34 [73.5%],
P=0.06) and less persistent candidemia (2/38 [5.3%] vs. 10/51 [19.6%],
P=0.05). There was no significant difference in 30-day mortality (60.5% vs. 47.3%,
P=0.21).
More than half the patients with candidemia gave all-negative BDG results shortly before candidemia onset. Thus, the Goldstream BDG test for candidemia has low predictive value for candidemia. C. albicans was the only Candida species significantly associated with any-positive BDG results before candidemia onset, but its ability to predict candidemia was also low (57.1%).
Positive blood culture for
Candida species is the mainstay of candidemia diagnosis, but the sensitivity of blood culture is suboptimal, and cultures may take several days to become positive [
5]. Studies have evaluated the BDG test as an alternative diagnostic method; the sensitivities varied from 47% to 95%, perhaps due to differences in study design, patient population, or test method [
4,
8,
9]. One study using the same kit as we used reported a sensitivity of 80% [
10]. Overall, 59.1% of our patients with proven candidemia gave all-negative BDG results within seven days prior to candidemia onset, a substantially higher proportion than those reported in the previous studies. Given the low predictive value of the BDG test used in our study, negative BDG results should be interpreted with caution and should not be used to exclude the possibility of invasive candidiasis.
The BDG results may have been influenced by previous use of systemic antifungals or the level of systemic fungal burden [
11,
12]. However, there was no difference in previous antifungal therapy between patients with any-positive BDG results and those with all-negative BDG results.
Predicting candidemia is especially important in critically ill patients who are at high risk of candidemia and have a high mortality rate. Therefore, it may be useful to use the
Candida score or colonization index in combination with the BDG test [
13,
14].
Different levels of association between
Candida species and any-positive BDG results have been reported [
15,
16], and our data support previous evidence [
17] of a positive association between
C. albicans and positive BDG results (
P=0.04).
This study had some limitations. First, because it was a single center-based investigation, our findings may not apply to populations with other candidemia prevalence or other tests. The Goldstream Fungus kit, which we used, is less studied than the Fungitell test (Associates of Cape Cod, Falmouth, MA, USA) [
9,
18]. Lastly, this was a retrospective study; the BDG tests were not performed at regular intervals, and test numbers varied among patients.
In conclusion, because of its limited sensitivity before candidemia onset, the Goldstream Fungus BDG test appears to be less reliable than anticipated from prior research. Hence, negative BDG values should be interpreted with caution in patients at high risk of invasive candidiasis.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download