INTRODUCTION
METHODS
Bacterial strains and phenotypes
Table 1
Abbreviations: AD, agar dilution; BMD, broth microdilution; ET, E-test; MIC, minimal inhibitory concentration; ST, sequence type; SCCmec, staphylococcal cassette chromosome mec; spa, staphylococcal protein A; VSSA, vancomycin-susceptible Staphylococcus aureus; VISA, vancomycin-intermediate resistant S. aureus.
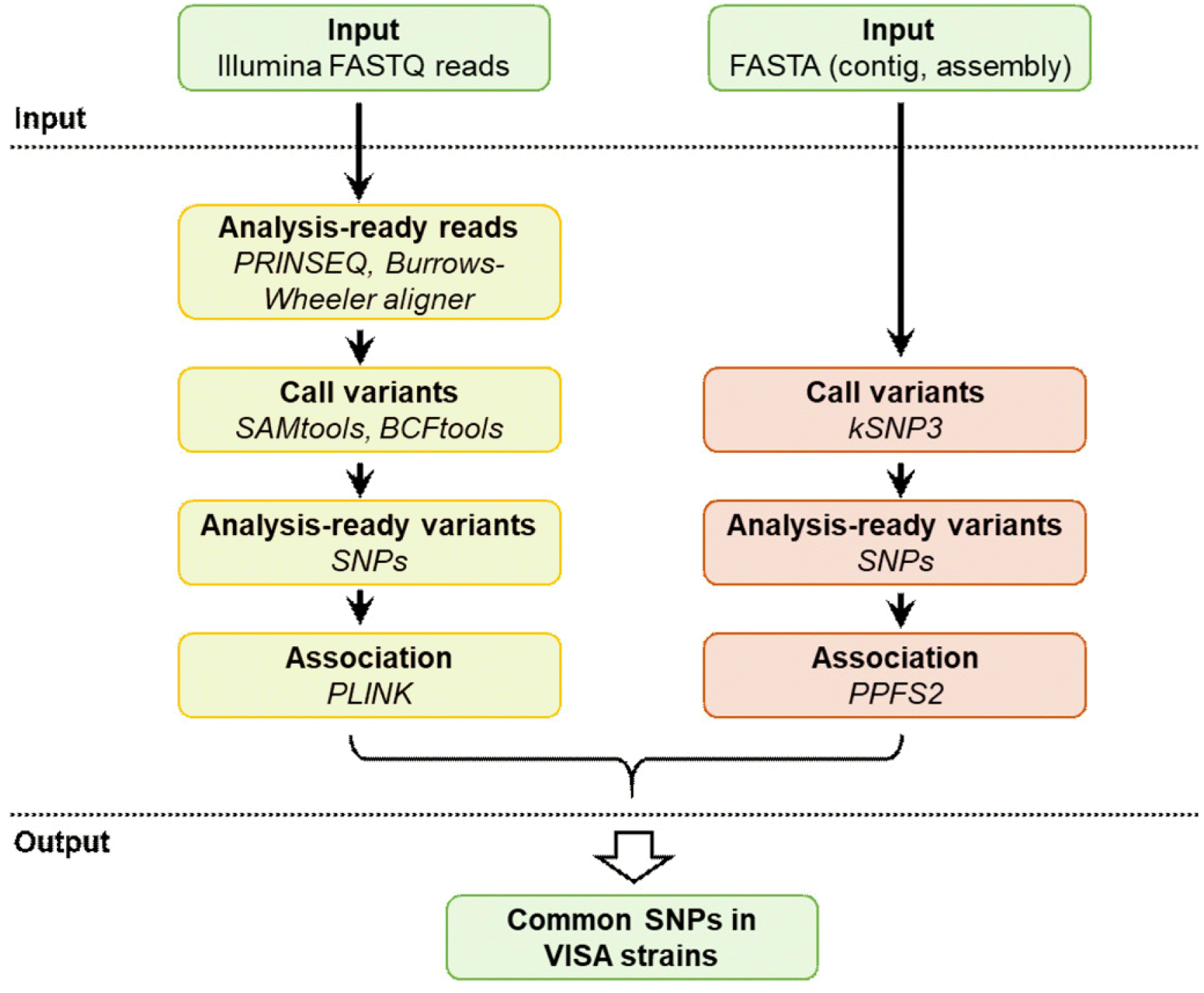
Whole-genome sequencing (WGS)
SNP calling and mGWAS
TaqMan SNP genotyping assay development and assessment
RESULTS
Loci associated with reduced vancomycin susceptibility
Table 2
| N315 locus tag* | Position | Mutation | Amino-acid change† | Biological function | Proportion of SNPs in VISA (%) |
|---|---|---|---|---|---|
| SA_RS01235 → | 249,622 | G→A | G203S | Gfo/Idh/MocA family oxidoreductase | 85.7 |
| SA_RS01705 ← / → SA_RS01710 | 350,179 | A→C | Intergenic (–335/–249) | Branched-chain amino acid transporter II carrier Protein/5´-nucleotidase | 78.6 |
| SA_RS01765 ← | 362,625 | G→C | S55T | RpiR family transcriptional regulator | 78.6 |
| SA_RS01845 ← | 378,175 | C→A | P530H | Hypothetical protein | 85.7 |
| SA_RS01985 ← / → SA_RS01990 | 407,772 | A→C | Intergenic (–302/–360) | Cystathionine gamma synthase/chromosome-partitioning protein ParB | 78.6 |
| SA_RS02445 → | 493,702 | A→G | A1013A | Glutamate synthase | 82.1 |
| SA_RS02450 → | 495,666 | A→G | P162P | Glutamate synthase subunit beta | 85.7 |
| SA_RS02640 → | 528,901 | G→A | E112E | Ribose phosphate pyrophosphokinase | 78.6 |
| SA_RS02980 → | 596,506 | T→C | A137A | Ribulokinase | 85.7 |
| SA_RS03020 → / → SA_RS03025 | 605,117 | C→T | Intergenic (+331/–98) | FMN-dependent NADPH-azoreductase/serine aspartate repeat-containing protein C | 78.6 |
| SA_RS03830 → / → SA_RS03835 | 765,823 | A→T | Intergenic (+57/–417) | Allophanate hydrolase/lipoteichoic acid synthase | 78.6 |
| SA_RS03930 → | 788,363 | T→C | G122G | Iron ABC transporter permease | 85.7 |
| SA_RS04890 → | 978,290 | C→T | S36L | GTP pyrophosphokinase | 78.6 |
| SA_RS04980 → | 1,001,999 | A→T | M354L | 2´,3´-Cyclic nucleotide 2´-phosphodiesterase | 85.7 |
| SA_RS05400 → | 1,081,200 | A→T | E163V | Chitinase | 78.6 |
| SA_RS05685 → / → SA_RS05690 | 1,135,543 | G→A | Intergenic (+41/–219) | Membrane protein/fibrinogen-binding protein | 78.6 |
| glpK → | 1,298,748 | G→A | E371K | Glycerol kinase | 78.6 |
| SA_RS06580 → | 1,320,949 | G→A | D189N | LuxR family transcriptional regulator | 78.6 |
| ebh ← | 1,446,514 | T→C | H3852H | Extracellular matrix-binding protein | 85.7 |
| SA_RS07360 ← / ← SA_RS07365 | 1,507,988 | G→A | Intergenic (–62/+30) | Nucleoside diphosphate kinase/heptaprenyl diphosphate synthase subunit II | 78.6 |
| SA_RS07605 ← | 1,551,898 | G→A | R24H | 6-Phosphogluconate dehydrogenase decarboxylating | 85.7 |
| SA_RS07790 ← | 1,582,579 | C→T | G194G | glucokinase | 78.6 |
| dnaK ← | 1,615,059 | C→T | N96N | Molecular chaperone DnaK | 78.6 |
| SA_RS08080 ← | 1,636,342 | T→C | I63T | Iron transporter | 85.7 |
| SA_RS08520 ← | 1,723,724 | A→T | K335I | DNA polymerase I | 78.6 |
| SA_RS08560 ← | 1,734,746 | A→G | G423G | Pyruvate kinase | 78.6 |
| SA_RS08765 ← | 1,781,936 | G→A | G56R | Acetyl-CoA synthetase | 85.7 |
| SA_RS08780 ← | 1,784,869 | A→T | E105D | Catabolite control protein A | 78.6 |
| SA_RS08975 ← | 1,833,017 | C→T | R119C | Autolysin | 85.7 |
| SA_RS09210 ← | 1,870,103 | C→T | T43I | Leucotoxin LukDv | 78.6 |
| SA_RS09725 ← | 1,942,181 | T→C | V171A | Acyl-CoA hydrolase | 85.7 |
| SA_RS09905 → / ← SA_RS09910 | 1,977,756 | C→A | Intergenic (+112/+180) | Staphostatin A/hypothetical protein | 78.6 |
| SA_RS10425 → | 2,055,468 | T→A | L212M | Potassium transporter KtrB | 85.7 |
| SA_RS10915 ← | 2,149,914 | G→A | E20K | Aminopyrimidine aminohydrolase | 78.6 |
| SA_RS11170 ← | 2,194,608 | C→T | E295K | Mannose 6 phosphate isomerase | 85.7 |
| SA_RS11400 ← | 2,248,253 | A→T | T233T | ABC transporter substrate-binding protein | 78.6 |
| SA_RS11550 → | 2,278,530 | A→G | T92A | Toxin | 78.6 |
| SA_RS11870 ← | 2,327,335 | A→G | K114E | Cyclic pyranopterin monophosphate synthase | 85.7 |
| SA_RS12195 ← | 2,386,988 | C→A | P352Q | Urocanate hydratase | 85.7 |
| SA_RS12200 → | 2,388,608 | T→C | L136S | LysR family transcriptional regulator | 85.7 |
| SA_RS12250 ← | 2,397,878 | A→T | I48F | Sodium ABC transporter ATP-binding protein | 85.7 |
| SA_RS12550 ← | 2,458,970 | G→C | G478A | Nitrite reductase | 85.7 |
| SA_RS13145 ← / ← SA_RS13150 | 2,575,425 | C→A | Intergenic (–85/+49) | Hypothetical protein/gluconate permease | 78.6 |
| SA_RS13485 ← / ← SA_RS13490 | 2,646,865 | A→T | Intergenic (–16/+497) | O-acetyltransferase OatA/GNAT family acetyltransferase | 78.6 |
| SA_RS13735 ← | 2,689,707 | C→T | P294L | Malate:quinone oxidoreductase | 78.6 |
| SA_RS13995 ← | 2,748,292 | G→T | R524S | Accessory Sec system translocase SecA2 | 78.6 |
Abbreviations: SNP, single-nucleotide polymorphism; VISA, vancomycin-intermediate resistant Staphylococcus aureus; FMN, flavin mononucletotide; ABC, ATP-binding cassette; ATP, adenosine triphosphate; GTP, guanosine triphosphate; GNAT, Gnc5-related N-acetyltransferase; SecA2, protein translocase subunit SecA2.
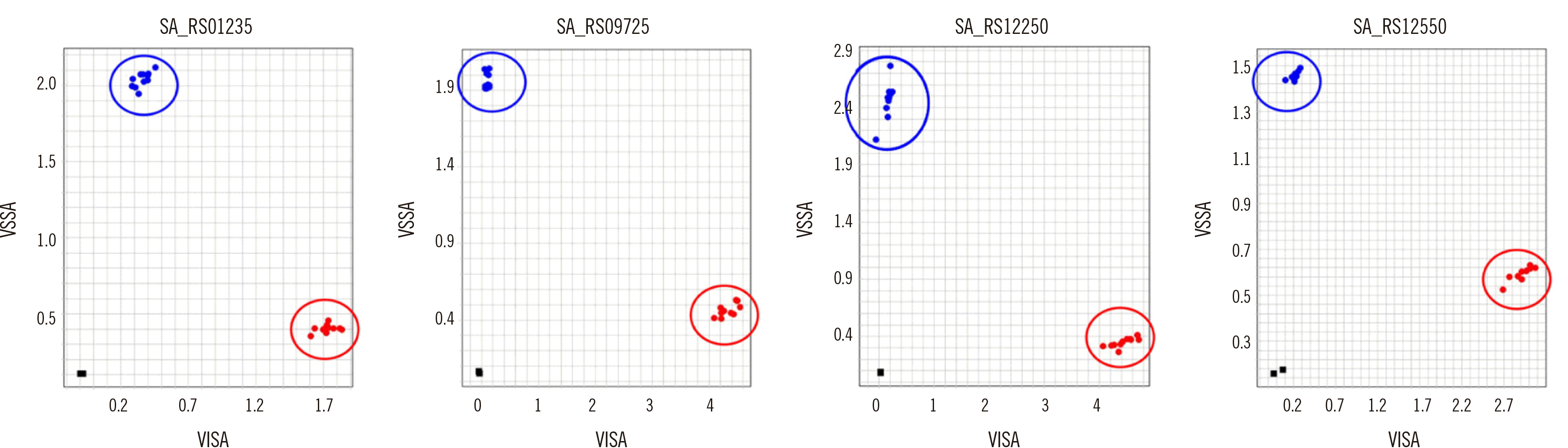
Rapid detection of SNPs associated with reduced vancomycin susceptibility
Fig. 2




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download