Abstract
Objectives
Materials and Methods
Results
Conclusion
Notes
Authors’ Contributions
All authors read and approved the final manuscript. K.R.M. and M.Y.E. collected the data and wrote the manuscript, J.Y.L., H.M., and M.H.S. revised and corrected the manuscript, and S.M.K. wrote the manuscript.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1I1A1A01058939).
Ethics Approval and Consent to Participate
This retrospective data analysis was approved by the Institutional Review Board of Seoul National University (No. S-D20200007) and informed consent was obtained from patients. All methods were performed in accordance with the relevant guidelines and regulations of the Declaration of Helsinki.
References
Fig. 1
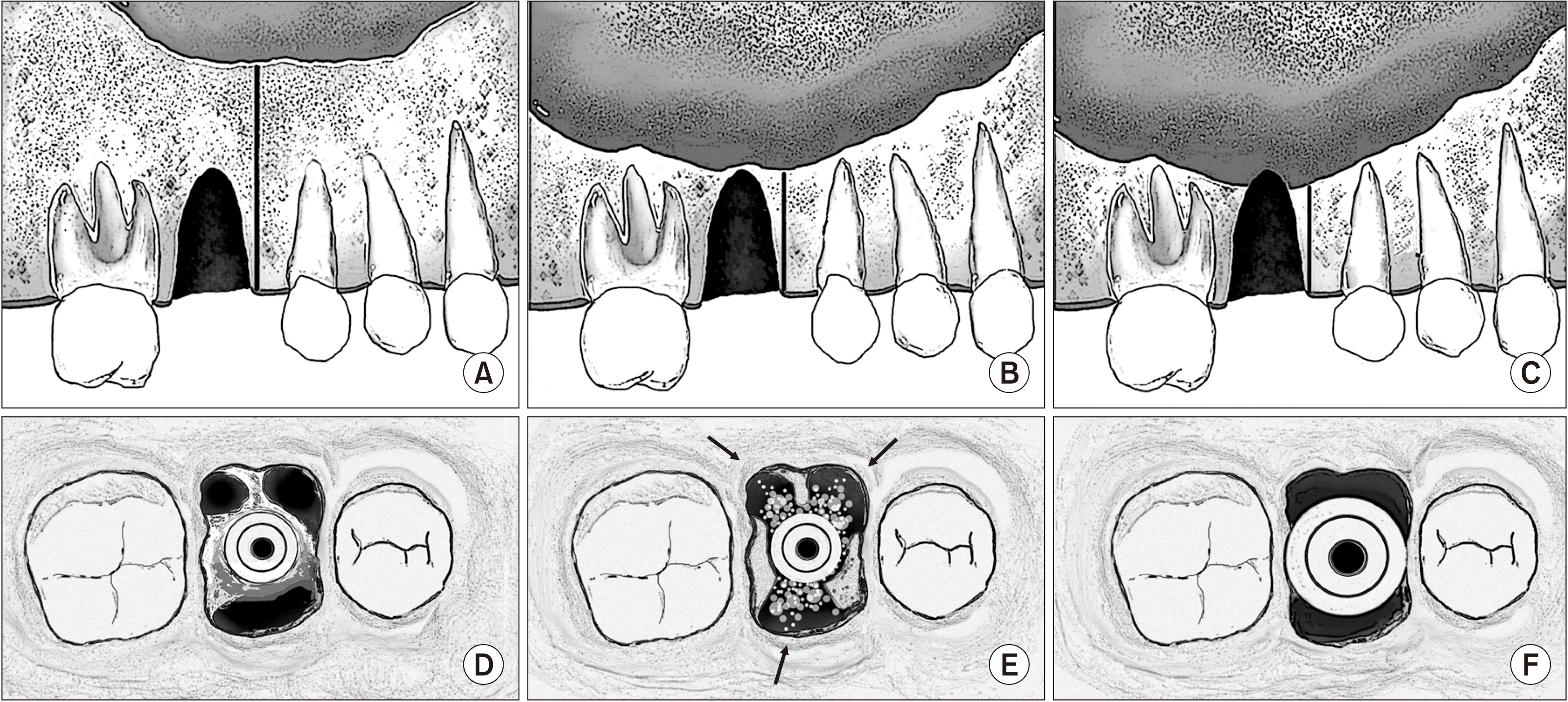
Fig. 2
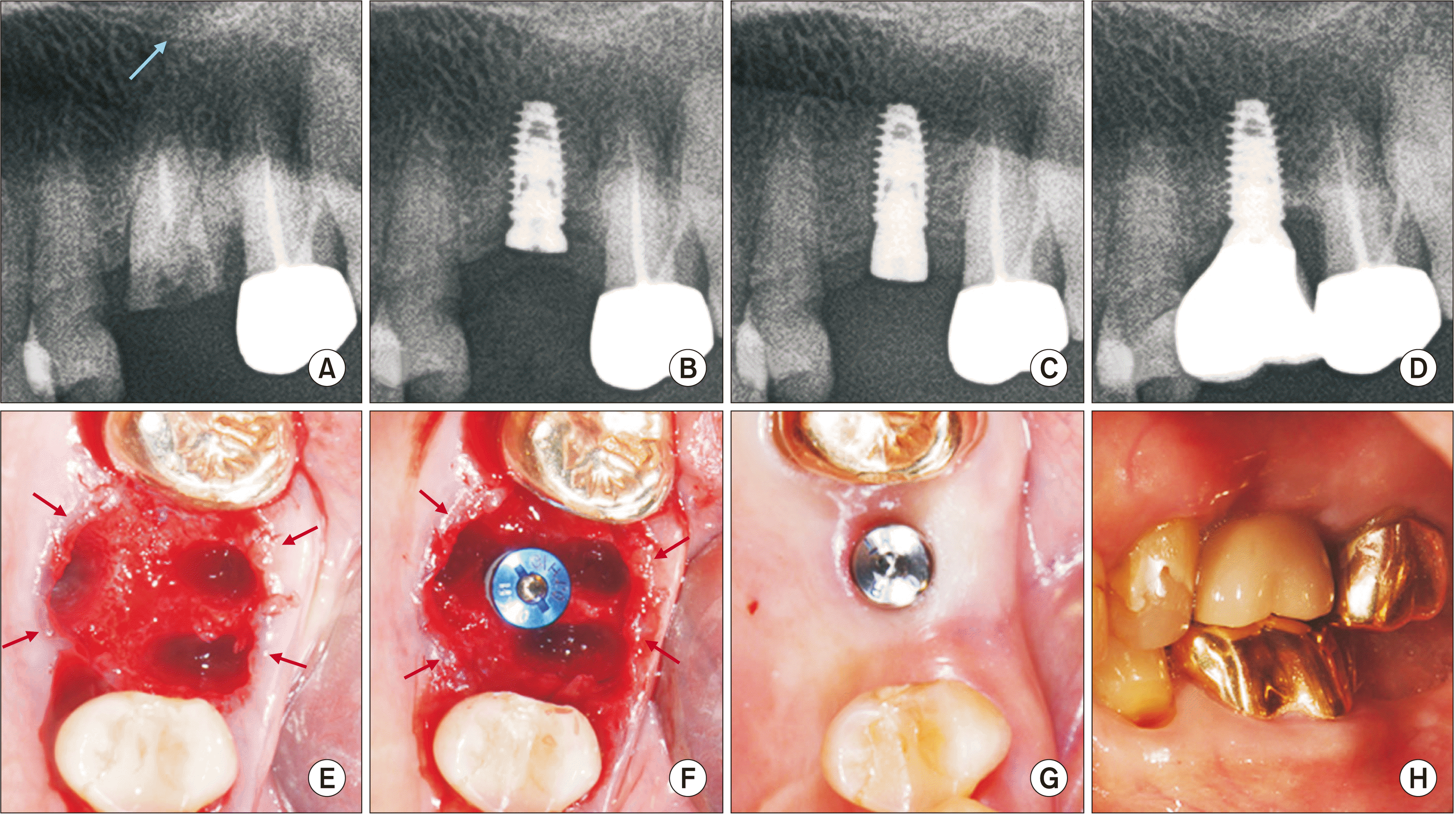
Fig. 3
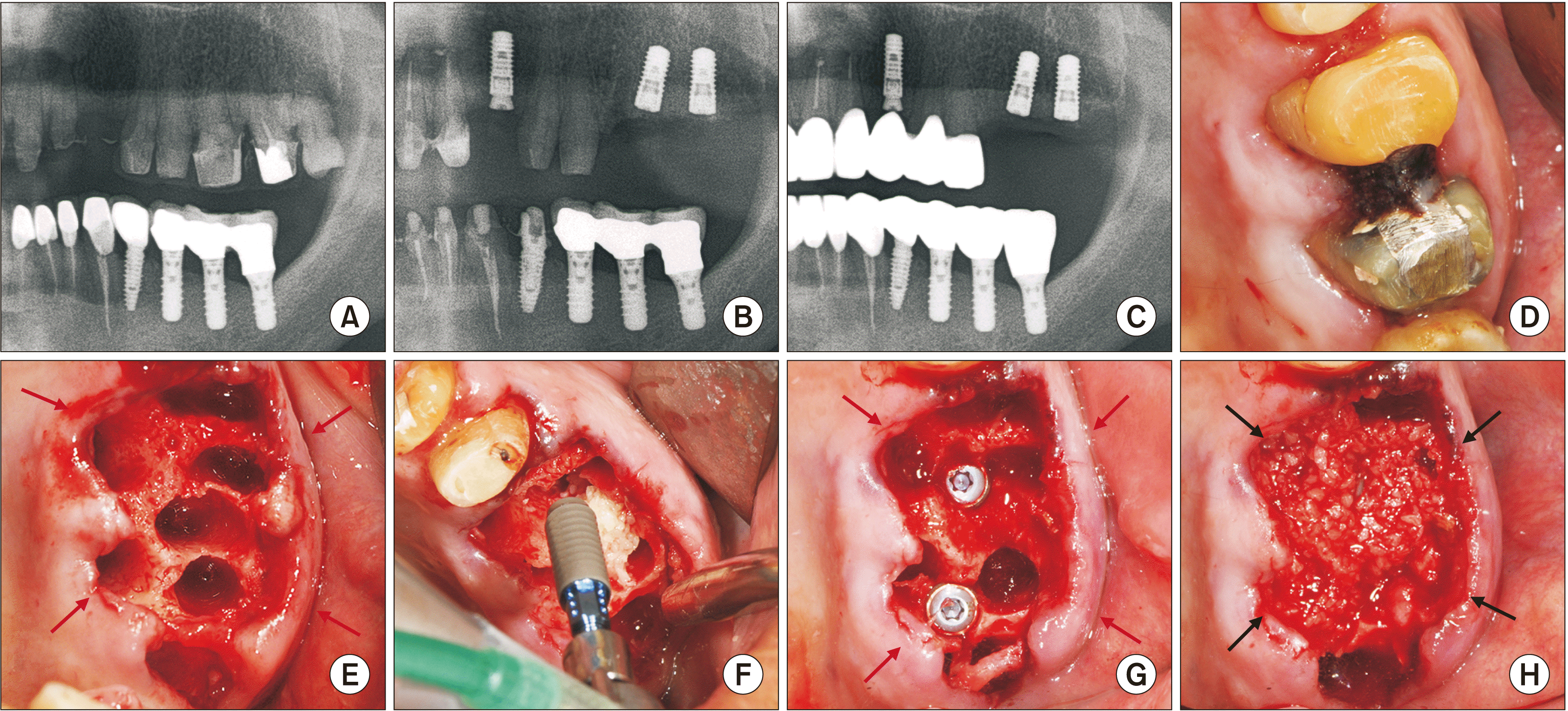
Fig. 4
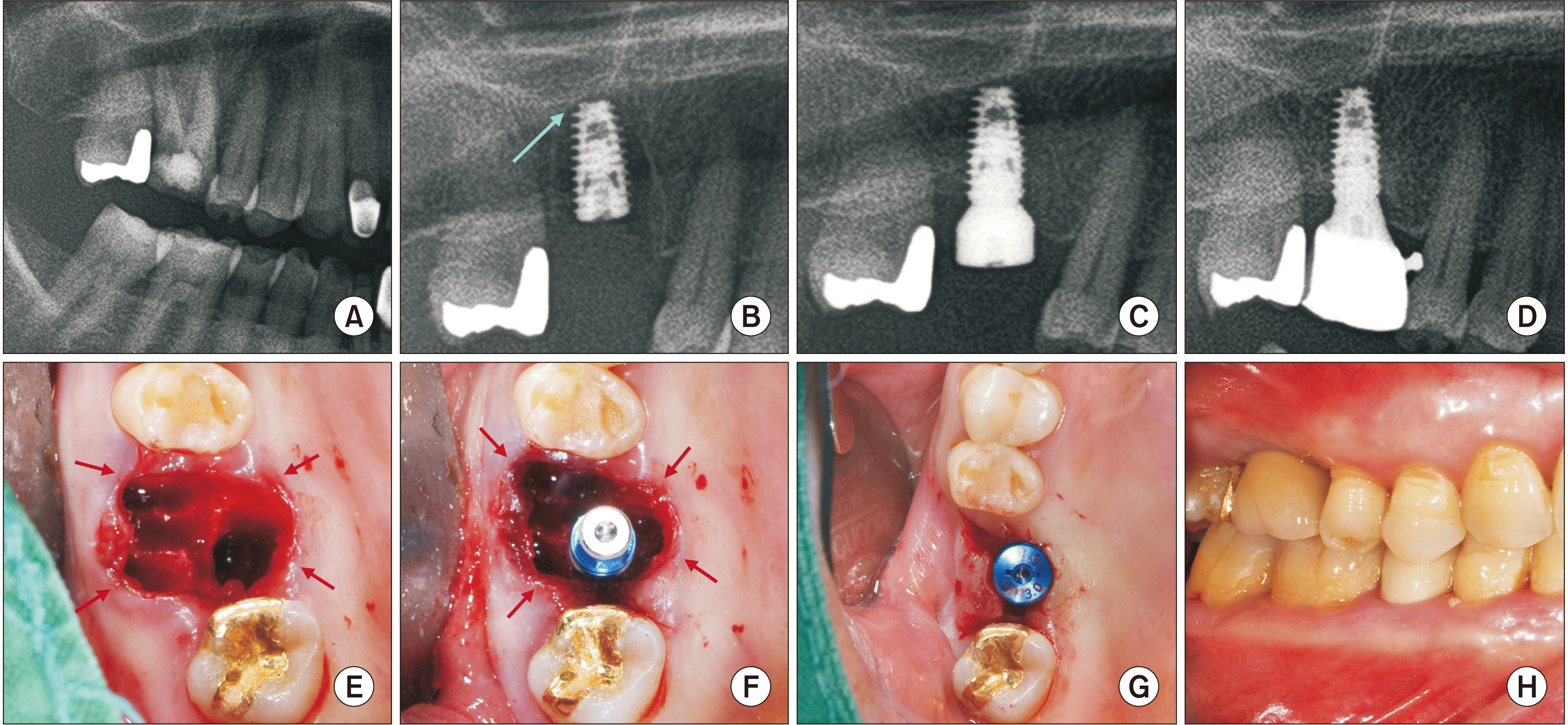
Fig. 5

Fig. 6
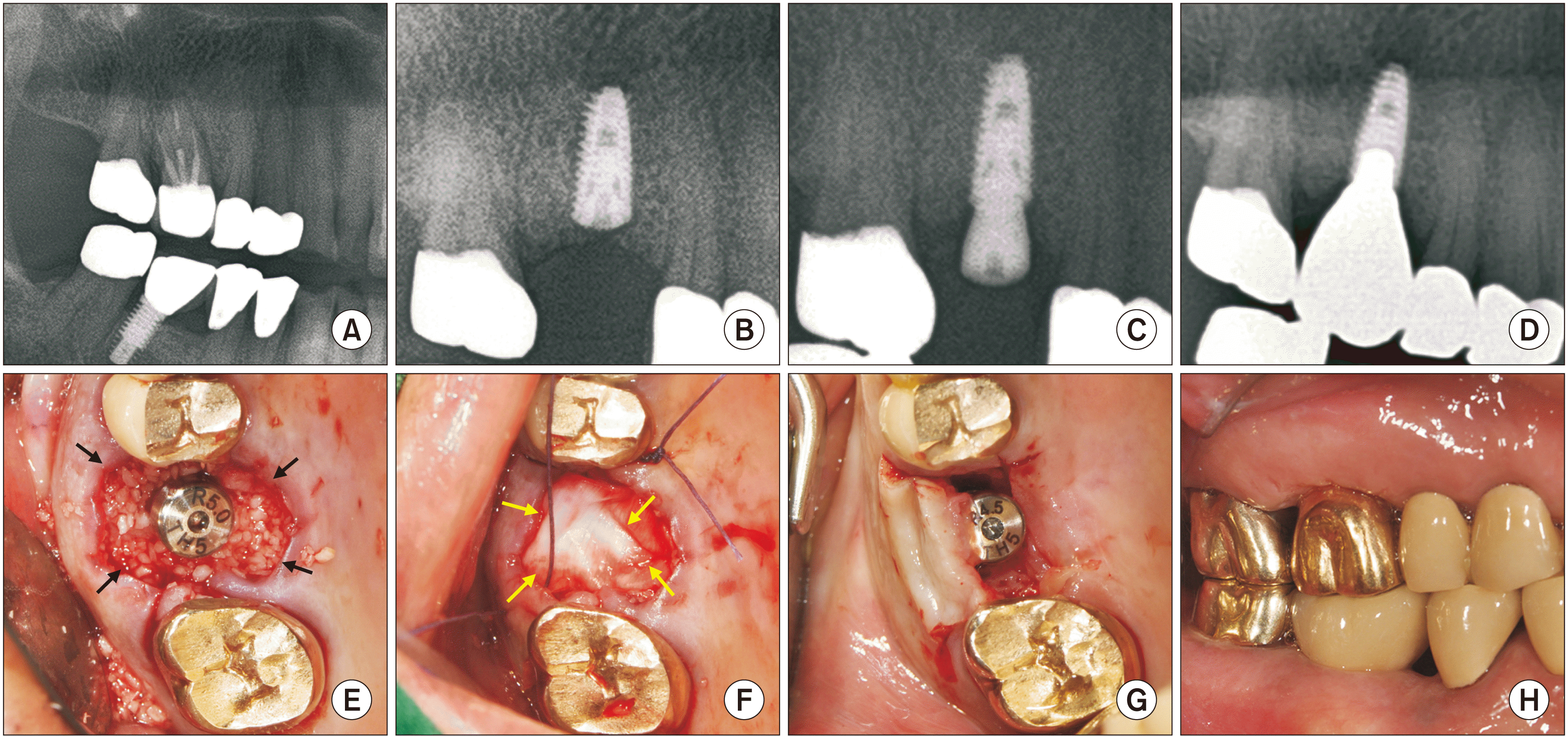
Fig. 7
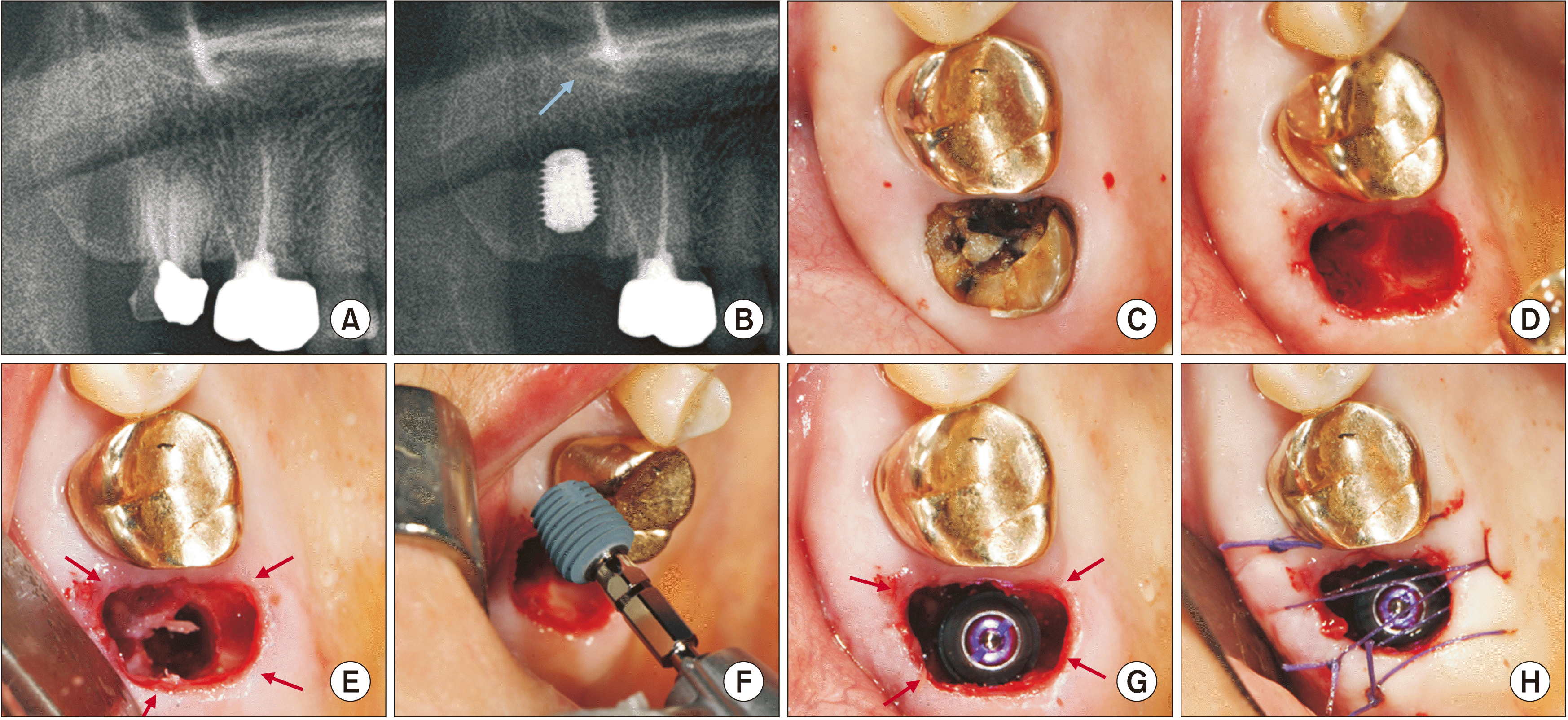
Table 1
| No. | Sex | Age (yr) | Tooth position | Implant type | Diameter (mm) | Length (mm) | SLA brand |
|---|---|---|---|---|---|---|---|
| 1 | M | 71 | #26 | TL | 4.1 | 10 | Straumann |
| 2 | F | 54 | #17 | TL | 4.1 | 8 | Straumann |
| #26 | TL | 4.1 | 8 | Straumann | |||
| #16 | TL | 4.1 | 10 | Straumann | |||
| 3 | F | 77 | #27 | TL | 4.1 | 6 | Straumann |
| 4 | M | 52 | #26 | TL | - | - | Straumann |
| 5 | M | 43 | #26 | TL | - | - | Straumann |
| 6 | M | 75 | #27 | TL | 4.1 | 8 | Straumann |
| 7 | F | 61 | #27 | BL | 4 | 10 | Luna |
| 8 | F | 54 | #17 | TL | - | - | Straumann |
| 9 | M | 72 | #16 | BL | - | - | Straumann |
| 10 | F | 75 | #16 | BL | 4 | 8.5 | Luna |
| 11 | F | 58 | #17 | BL | 4 | 8.5 | Luna |
| 12 | F | 70 | #26 | BL | - | - | Straumann |
| 13 | F | 62 | #16 | BL | 3.3 | 8 | Straumann |
| 14 | F | 67 | #26 | BL | 4 | 8.5 | Luna |
| 15 | F | 67 | #26 | BL | 4 | 8.5 | Luna |
| 16 | M | 71 | #16 | BL | 4 | 8.5 | Luna |
| 17 | F | 71 | #16 (re-install) | BL | 4 (5)1 | 7 (7)1 | Luna |
| 18 | M | 64 | #16 (re-install) | BL | 4 (4.5)1 | 7 (7)1 | Luna |
| 19 | M | 78 | #16 | BL | 4.5 | 10 | Luna |
| 20 | F | 16 | #16 | BL | 4 | 7 | Luna |
| 21 | F | 76 | #16 | BL | 4 | 8.5 | Luna |
| 22 | M | 60 | #26 | BL | 4 | 8.5 | Luna |
| 23 | F | 62 | #17 | BL | 6.5 | 8.5 | Anyone |
| 24 | F | 72 | #26 | BL | 4 | 10 | Luna |
| 25 | M | 73 | #26 | BL | 4.1 | 10 | Straumann |
| #27 | BL | 4.1 | 10 | Straumann | |||
| 26 | M | 86 | #17 | BL | 4 | 7 | Luna |
Table 2
| MBL from installation (mm)1 | MBL from previous follow-up visit (mm)2 | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Mesial | Distal | Mesial | Distal | ||
| T1 | –0.44±0.62 | 0.35±0.72 | - | - | |
| T2 | 0.14±0.66 | –0.27±0.52 | 0.30±0.52 | –0.71±0.78 | |
| T3 | 0.20±0.36 | –0.16±0.60 | 0.01±0.42 | 0.20±0.14 | |
| T4 | –0.02±0.02 | –0.28±0.52 | 0.03±0.03 | 0.01±0.01 | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download