I. Introduction
Dental implants have been a reliable treatment option for rehabilitation of completely and partially edentulous patients. In addition to biocompatibility, the durability of implant fixtures is a major concern for dentists and patients. Mechanical complications of the implant include abutment screw loosening, screw fracture, loss of implant prostheses, and implant fracture. Although the success rate of implants is greater than 90%
1, the incidence of implant fractures has been reported to range from 0.16% to 1.5% of cases
2. One of the major causes of implant fracture is biomechanical overloading, which occurs due to various parafunctional activities like bruxism, inadequate occlusion, and distal extensions or cantilevers
3-5. Other causes are peri-implant vertical bone loss due to peri-implantitis and occlusal trauma, with galvanic corrosion as an additional causative factor contributing to implant fractures
6. Management of cases of screw and implant fractures can pose a challenge to clinicians because of its surgical implications. In addition, bone preservation at the implant socket site and rehabilitation after implant removal are essential.
Wee and Lee
7 reported a case of implant fracture after screw fracture in a tissue level implant and suggested that screw fracture could be a precursor to fixture fracture. Reports of cases of simultaneous screw fracture and implant damage are rare. Recent studies have also questioned the importance of implant surface chemical composition. However, the available information on the release of elements from the fractured implant retrieved from the human body was very little. It is hypothesized that surface contamination might initiate the inflammatory response and possibly provoke the dissolution of titanium
8. The screw loosening and the micro-movement might enhance this dissolving process and alter the implant surface and finally compromise the osseointegration. Studying the presence of dissolving ions on the fixture surface and peri-implant tissue might reveal the corrosion level during micro movement, and give the suggestion for further study of the multifactor corrosion phenomenon in implants that are failed due to fracture and other various etiologies.
Investigators have reported different techniques to determine the presence of elemental composition of the implant surface and integrated bone. Some studies evaluated the failed implant surface using X-ray photoelectron spectroscopy and Auger electron spectroscopy
9,10. In our study, the element composition analysis was performed using the energy dispersive X-ray spectroscopy (EDS), which has relatively high energy and deep penetration into the sample surface
11. With the EDS equipment, the compositional information can be achieved over a depth of 1 µm, which gives more information about the implant surface below the organic matter and titanium oxide layer.
This case report aims to describe the management of a case of fixture damage that occurred with screw fracture in a tissue level implant and microscopic evaluation of the failure implant segment using scanning electron microscopy (SEM) and EDS.
Go to :

III. Discussion
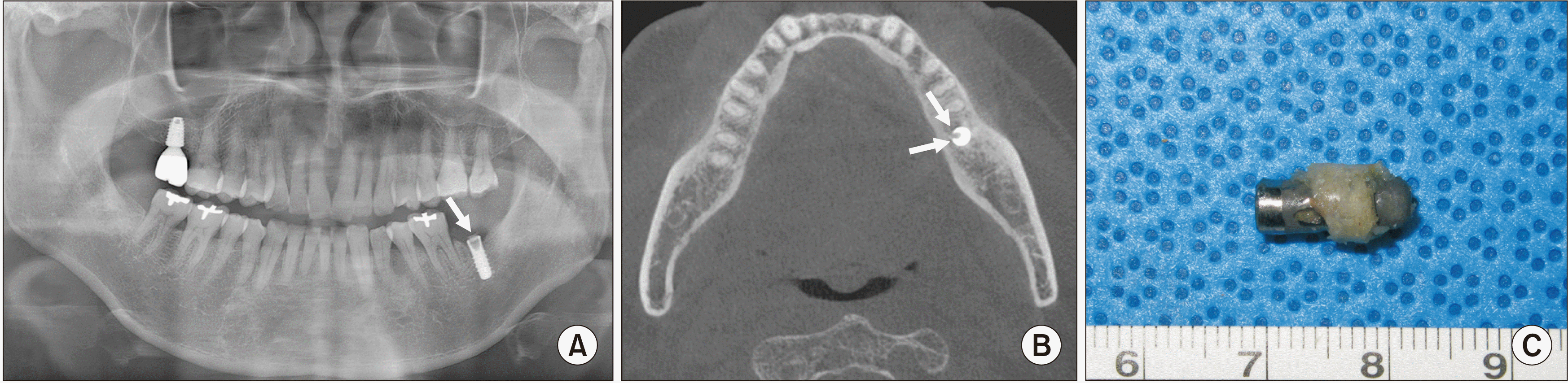
In the current case, the failed implant was a tissue-level implant from the ITI system (Straumann implant; Institut Straumann AG, Basel, Switzerland). Tissue-level implants are designed to reduce marginal bone loss after implant placement by placing the platform 1.8 or 2.8 mm above bone level. Some studies have reported that marginal bone loss is associated with screw and fixture fractures
12,13. With the thick wall design, which can withstand heavy occlusal forces, tissue level implants are expected to be resistant to fracture. However, there are no studies comparing the prevalence of fractures between tissue-level and bone-level implants. Risk factors for implant fractures include type of prosthetic structure (single crown), presence of parafunction, inappropriate occlusion, marginal bone resorption, and screw loosening.
The SEM-EDS results revealed an image of a fractured fixture with both normal and irregular bone patterns. Microfractures and scratching lines were observed in the adjacent area of the broken area of the fixture, indicating a fatigue condition of the fixture, which might have been caused by screw loosening and its movement during function over a long period of time. The elemental distribution map revealed a homogeneous oxidized Ti surface with particles composed of C and Si. Most of the integrated bone layer on the fixture surface was of normal bone structure with bone lacunae. However, irregular bone structure with the presence of organic matter also was detected. In addition to major elements, there was the detection of metal and non-metal elements including N, Na, F, Si, Al, and Au. The C, N, and Si were suggested to be absorbed during the manufacture. In addition, Si contamination might have originated from the glass storage vials. The rubber gloves could be a source of Si and C, but the possibility is not high
11. The Al could be residual from sandblasting surface treatment, while Na and F could have been absorbed from the intraoral environment. Noticeable, the level of Au on the implant surface and peri-implant tissue was significantly high in the middle and apical region of the implant. The Au ions were suggested to be derived from the corrosion process of the prosthetic, however, this phenomenon needs to be further studied for confirmation.
Shemtov-Yona and Rittel
14 performed an SEM-EDS study for chemical composition identification of commercial pure Ti (CP-Ti) and Ti-6Al-4V retrieved from fractured dental implants. The authors reported that the fracture surfaces were covered with different layers, including the Ti surface of the fixture, organic layer (containing mainly C), and inorganic layer (containing mainly Ca and P, suggestive of bone material). It is theorized that metal fatigue is the main failure mechanism of a fractured implant, and the intra-oral environment might cause a significant reduction in implant fatigue performance
14. Furthermore, abrasion on the surfaces of the implant-abutment connection during masticatory tooth movement can create wear particles
8. Due to damage of the titanium oxide layer, the exposed fresh titanium surface reacts immediately with the environment, corresponding to an anodic partial current and a subsequent increase of the corrosion rate due to the high chemical reactivity of the bare metal
8. The mechanical instability of implant-abutment connections appears to trigger a chain of synergistic negative events involving issues such as micro-gap widening, micromovements of the contacting surfaces, wear, ion and debris release, peri-implant inflammatory reactions, and peri-implantitis
15. In this study, the turnover bone structures were detected, and trace amounts of corrosion metals such as Au and Ti in the bone tissue were recorded, which might have resulted from instability and micro-movement of the implant-abutment connection over an extended period of time. Further,
in vivo and
in vitro evidence is needed for confirmation of this phenomenon.
It is worth noting that screw fracture occurred at the same time as fixture fracture in this case. In screw-retained implants, the stress produced by the micro-movement of screws can create constant tension on the implant fixture. Therefore, screw loosening often causes implant fracture and can be considered a warning signal. In this case, the patient did not have the recall check-up for a long period, and the continuous use of the prosthesis with loosening screw might be the main cause of the implant fracture. This implies the importance of periodic maintenance. The clinician also should instruct the patient to visit the clinic as soon as whenever there is any sign of mobility of the prosthesis. Once the fracture happens and the failure implant is removed, re-installation of an implant with wider diameter can be performed immediately after implant removal. However, in cases explantation using trephine bur, the socket should be preserved with an allogeneic bone graft and a wide implant can be used to maximize the potential initial stability. Long-term periodic follow-up is essential and the prosthesis, abutment screw, and peri-implant tissue should be carefully examined to detect and prevent any biomechanical complications.
In conclusion, dental implant fracture is an infrequent but important cause of implant therapy failure. The SEM and EDS results from in current study give an enlightenment of the failed fixture surface micromorphology with microfracture and contaminated chemical compositions. Noticeably, the significantly high level of Au on the implant surface and the trace amounts of corrosion metals such as Au and Ti in the bone tissue were recorded, which might have resulted from instability and micro-movement of the implant-abutment connection over an extended period of time. Further study with larger number of patient and different types of implants is needed for further conclusion.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download