Abstract
Objective
The goal of this study was to analyze the clinical outcomes of endoscopic third ventriculostomy (ETV) and endoscopic septostomy when shunt malfunction occurs in a patient who has previously undergone placement of a ventriculoperitoneal shunt.
Methods
From 2001 to 2020 at Seoul National University Children's Hospital, patients who underwent ETV or endoscopic septostomy for shunt malfunction were retrospectively analyzed. Initial diagnosis (etiology of hydrocephalus), age at first shunt insertion, age at endoscopic procedure, magnetic resonance or computed tomography image, subsequent shunting data, and follow-up period were included.
Results
Thirty-six patients were included in this retrospective study. Twenty-nine patients, 18 males and 11 females, with shunt malfunction underwent ETV. At the time of shunting, the age ranged from 1 day to 15.4 years (mean, 2.4 years). The mean age at the time of ETV was 13.1 years (range, 0.7 to 29.6 years). Nineteen patients remained shunt revision free. The 5-year shunt revisionfree survival rate was 69% (95% confidence interval [CI], 0.54–0.88). Seven patients, three males and four females, with shunt malfunction underwent endoscopic septostomy. At the time of shunting, the age ranged from 0.2 to 12 years (mean, 3.9 years). The mean age at the time of endoscopic septostomy was 11.9 years (range, 0.5 to 29.5 years). Four patients remained free of shunt revision or addition. The 5-year shunt revision-free survival rate was 57% (95% CI, 0.3–1.0). There were no complications associated with the endoscopic procedures.
Shunting is a surgical method that has long been used to insert conduits to drain cerebrospinal fluid (CSF) from the brain and move it to other spaces of the body. Shunting can lead to rapid improvement of symptoms in most patients but requires continuous close attention and care. Infection, obstruction or blockage of the shunt device are the biggest problems. To prevent these device-related problems, endoscopic procedures can be used instead of shunting. ETV is considered the treatment of choice for obstructive hydrocephalus. To do this, surgeons perforate the floor of the third ventricle and create a communication between the ventricle and the subarachnoid space (interpeduncular cistern). However, there are some restrictions on surgical indications depending on the age of the patient, the etiology of hydrocephalus, and the anatomical structure of the brain. Approximately 75% of patients with good indications (more than 1 year of age, obstructive hydrocephalus, no previous shunt) reported that hydrocephalus had been resolved. However, the success rate in patients without good indications is less than 50% [11]. Nevertheless, there is a great advantage of using a natural CSF circulation process without foreign body (shunt device) insertion.
Problems (malfunctions and infections) with existing shunt devices are usually resolved by shunt revision, but in some cases, endoscopic procedures can help resolve the problem and do not require a shunt revision procedure.
The aim of this study was to investigate the success rate and safety of endoscopic procedures when shunt failure occurred in patients who had undergone ventriculoperitoneal (VP) shunt operations.
The study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 2101- 126-1190). Patient consent was not required given that this study only involved a review of the medical record.
This study is a single-center, consecutive, nonrandomized, retrospective, case series reviewing the electronic medical records of patients using endoscopic procedures for VP shunt malfunction at the Seoul National University Children’s Hospital. The study period was 19 years between July 2001 and May 2020, and a total of 36 patients were identified using coding for ETV and endoscopic septostomy after VP shunt operations. Only patients with increased intracranial pressure (ICP) signs or symptoms were included in this study, and those with ventriculomegaly only on images were excluded. The Wolf rigid ventriculoendoscope system (2.7 mm, 0-degree; Richard Wolf GmbH, Knittlingen, Germany) was used in the procedures. We have been using a cranial electromagnetic surgical navigation system (StealthStation® AxiEM™; Medtronic, Louisville, CO, USA) since 2014. It allows for trajectory planning to avoid cortical vessel injury and minimize brain parenchymal damage.
The data collected included demographic information, preoperative assessment (etiology of hydrocephalus, reason for endoscopic procedure, and previous shunt duration), preoperative imaging, date of surgery, age at surgery, surgical details, and postoperative follow-up.
Preoperative magnetic resonance imaging (MRI) or computed tomography (CT) scans were performed on all patients with existing shunt problems. As previously described in the literature, we measured the anatomical features of the third ventricle. Third ventricular floor bowing was considered if the tuber cinereum was 3 mm lower than the reference line drawn from the optic chiasm to the highest point of the midbrain [12]. If the patient had third ventricular floor bowing, we considered ETV first. If third ventricular floor bowing was not clear, cine MR was performed (n=10). ETV was first considered over shunt revision in patients showing insufficient flow in the aqueduct of Sylvius or fourth ventricle outlet on cine MR.
The indication for endoscopic septostomy was that preoperative MRI or CT scans demonstrated isolated ventricular enlargement. The ipsilateral lateral ventricle containing a shunt proximal catheter inside which was larger than the opposite lateral ventricle, and obstruction of the foramen of Monro was suspected. When performing septostomy, a burr hole was made 2–3 cm lateral to the Kocher's point. All patients who underwent endoscopic septostomy (n=7) had lateral ventricle isolation, and among them, two patients had multiple intraventricular septations. They needed additional fenestration.
Based on the patient’s medical records, the ETV success score (ETVSS) [22] was also analyzed to assess whether ETV success was predictable, even in a second procedure for a shunt problem.
Patients were classified into two groups based on their clinical courses. The ‘success’ result was defined as the resolution of clinical symptoms and signs of increased ICP or slowing of head growth rate. A ‘failure’ result was defined as a lack of improvement or the recurrence of clinical symptoms and signs of increased ICP or subsequent shunt revision.
The Mann-Whitney U test or Fisher’s exact test was used to compare differences between two independent groups. In addition, all results were calculated with the standard deviation and odds ratio (OR) of the 95% confidence interval (CI). The success rate and shunt revision rate of the endoscopic procedure were measured by the Kaplan-Meier method, and the difference was compared using the log rank test. Univariate logistic regression analysis of the endoscopic procedure was conducted with various factors as independent variables. The ETVSS was calculated as a continuous value for all patients, and the risk of failure was evaluated. All statistical analyses were performed using SPSS ver. 25.0 software (SPSS Inc., Chicago, IL, USA), R (version 3.6.1), and RStudio (version 1.4.1717), and a p-value <0.05 was considered statistically significant.
In this series, there were 36 patients, of which 21 (58%) were male and 15 (42%) were female. The age at shunt insertion ranged from 1 day to 15.4 years, with a mean age of 2.3 years. The mean follow-up duration was 3.0 years (range, 3 days to 18.1 years) after the endoscopic procedure.
Twenty-nine patients, 18 males and 11 females, with previous shunt operations underwent ETV (Table 1). At the time of shunt insertion, the age ranged from 1 day to 15.4 years (mean, 2.4 years). Twenty-one patients were under 1 year old when they underwent shunt operation. The mean age at the time of ETV was 13.1 years (range, 0.7 to 29.6 years). Three patients less than 1 year of age underwent ETV. The etiology of hydrocephalus was congenital aqueductal stenosis in seven cases and aqueductal compression by tumor in nine cases. Nine patients had suspected malabsorption or obstruction due to germinal matrix/intraventricular hemorrhage. Tuberculosis meningitis occurred in one case, and bacterial meningitis occurred in three cases. Of the 29 patients, four patients did not have a MRI performed. Twenty-three patients showed aqueductal flow obstruction, and two patients showed fourth ventricle outlet obstruction. Seventeen out of 25 patients were confirmed to have third ventricular floor bowing (Fig. 1). The mean of the third ventricular floor bowing was 5.3 mm below the reference line. Eight of 17 patients with third ventricular floor bowing failed ETV (success rate, 52.9%). Moreover, three of eight patients who failed ETV had CSF flow obstruction on cine MR before surgery. One in eight patients without third ventricular floor bowing failed ETV (success rate, 87.5%). All patients without third ventricular floor bowing but who were suspected to have CSF flow obstruction on cine MR (Fig. 2) showed 100% success in ETV. Nineteen patients (65.5%) remained shunt revision free. The 5-year shunt revision-free survival rate was 69% (95% CI, 0.54–0.88, Fig. 3A). The other 10 patients needed shunt revision.
Seven patients, three males and four females, with previous shunt operations underwent endoscopic septostomy. At the time of shunt insertion, the age ranged from 0.2 to 12 years (mean, 3.9 years). Four of the patients were under 1 year old when they underwent shunt operation. The mean age at the time of the endoscopic septostomy was 11.9 years (range, 0.5 to 29.5 years). Only one patient less than 1 year of age underwent an endoscopic septostomy. The etiology of hydrocephalus was congenital porencephaly in two cases and postoperative hydrocephalus in three cases (two tumor surgeries and one epilepsy surgery). One patient had suspected malabsorption or obstruction due to germinal matrix/intraventricular hemorrhage, and one patient had bacterial meningitis (Table 2). Four patients remained free of shunt revision or addition. The 5-year shunt revision-free survival rate was 57% (95% CI, 0.3–1.0; Fig. 3B). The other three patients needed shunt revision. All three cases of unilateral hydrocephalus at the initial shunt operation showed symptom improvement after septostomy. Two patients showed evidence of isolated lateral ventricle, one with a trapped trigone area and the other with a trapped fourth ventricle case. These two patients received endoscopic septostomy along with endoscopic fenestration to create passages for entrapment. None of them needed additional shunt revision or addition. No complications were associated with the endoscopic procedures.
Most endoscopic procedure failures occurred within a month. Seven patients needed shunt revision immediately after ETV. For patients who had a problem within a month, the mean time to shunt revision was 16 days (range, 3 to 28 days). Except for one patient, every failure occurred within 3 months. In the cases of endoscopic septostomy, two patients failed within a month, and one patient failed within 2 months. They needed shunt revision after an average of 23 days (range, 12 to 44 days). Similar to the ETV results, failure was revealed within a month or 2.
The univariate results are shown in Tables 3 and 4. The higher the OR was, the higher the probability of shunt revision. Age at shunt insertion, etiology of hydrocephalus, reason for ETV, previous shunt duration and ETVSS were analyzed (Table 5). No significant predictors of procedure success could be found in our study.
The VP shunt malfunction rate reaches 30% in the first year of placement and thereafter is approximately 10% per year. Sainte-Rose et al. [32] suggested that 81% of shunts require revision after 12 years. Foreign body insertion is vulnerable to infection, and shunt infection occurs 5–10% of the time [29]. In an effort to reduce these shunt-related complications, the demand for endoscopic surgery has increased.
Treatment with endoscopy is used as an initial treatment in patients with primary hydrocephalus, but it is also used to replace shunts or to avoid multiple shunts in patients with shunt malfunction or infection. In patients with shunts, the shunt system can be occluded and develop ventricular dilatation on the shunted side with suspected but unconfirmed obstruction of the foramen of Monro. Otherwise, the inflammatory process after germinal matrix hemorrhage within the ventricle, bacterial meningitis, encephalitis, shunt infection, trauma or intracranial surgery can cause entrapped ventricles.
Studies reporting ETV results after VP shunt malfunction show a success rate of 42–84% (Table 6). The overall pooled success rate was 68.2% [39]. The reported complication rate was 6.1% (0–15.9%). Studies including adult patients had relatively high ETV success rates [5,29,35,38]. In 2009, a multivariate analysis was conducted on 618 children in the UK, Canada, and Israel to predict the possibility of ETV success [22]. They reported that age, etiology, and previous shunt were the most important factors in predicting ETV success and suggested a way to calculate the success rate through the ETVSS. In our series, all the patients had previous shunts, and the overall ETVSS varied from 40 to 90, with a mean of 72.4, which is not far from the real success rate of ETV. Our success rate was 65.5%, which is similar to the results of other existing studies.
As Marton et al. [25] reported, age at ETV does not have a statistically significant effect on ETV success in our study population. Most ETV failures were revealed within the first month. Seven of 10 patients needed shunt revision immediately. Except for one patient, all failure results were confirmed within three months. The mean time to ETV failure was 16 days. Many studies have also reported that failure occurs within a month [8,24,27,29]. Many authors have reported that a previous etiology of hydrocephalus affects the success of ETV [15,25,29]. However, our data show that regardless of the etiology of hydrocephalus, whether congenital aqueductal stenosis, tumor-related, posthemorrhagic, or postinfectious, none of them affected the success of ETV. This result may be due to selective indications of endoscopic procedures when there are findings that may be suspected to be obstructive hydrocephalus, regardless of the etiology. If there were some indicators, such as third ventricular floor bowing on MRI or CSF flow obstruction on cine MR, the hydrocephalus may be considered an obstructive type. The number of patients in each group was small; thus, nothing was statistically significant. Previous shunt duration was not a factor influencing ETV success.
In the present study, we performed endoscopic septostomy on previously shunted patients with shunt malfunction when secondary obstruction of the foramen of Monro was suspected. Patients had an increased lateral ventricle on the side containing the shunt proximal catheter and a relatively normal contralateral ventricle size. It was obviously caused by shunt malfunction. However, obstruction of foramen of Monro was not confirmed.
Unilateral hydrocephalus is a rare form of hydrocephalus. It mainly develops after intraventricular hemorrhage or meningitis [14,20,33]. In patients with VP shunts, the causes include shunt infection, shunt overdrainage and damage to the ependymal lining during shunt catheter insertion [14,18,28,31]. However, in this study, shunt overdrainage can be excluded because the ipsilateral lateral ventricle containing the shunt catheter inside was larger than the opposite side of the lateral ventricle. It has various forms according to the obstruction site in the ventricular system. The traditional treatment of hydrocephalus with a previous VP shunt malfunction included adding or replacing the shunt [1,14,18,20]. However, multiple shunt insertions lead to higher rates of shunt failure or complication [23,37]. Therefore, neurosurgeons are trying to reduce the number of initial shunts and avoid additional shunts as much as possible by introducing endoscopic septostomy. Uncomplicated asymmetric hydrocephalus was successfully cured with endoscopy. Case series on endoscopic septostomy showed a success rate of 53–87% with asymmetric hydrocephalus [2,16,30]. In the present study, fenestration of the septum pellucidum with a previous VP shunt occlusion showed a success rate similar to that reported in the literature. In some literature, success was defined even if there had been multiple endoscopic procedures on the isolated ventricle. However, in our group, there were only two cases that were tried several times. We categorized the two cases as failures because they needed shunt insertion during the course of treatment. It was difficult to compare simple success rates because the complexity in the literature varies from patient to patient, and the etiology of isolated unilateral hydrocephalus was diverse.
Imaging can be considered at the surgeon’s discretion to assist with surgical planning. A constructive interference in steady state (CISS) study can clearly illustrate even paper-thin membranous obstruction within the aqueduct and fourth ventricle outlet. Since T2-cine MRI gives information on CSF flow, it is very helpful to perform it when obstruction is suspected. With these imaging techniques, we can also visualize the third ventricular floor bowing and the thin membranous structure within the isolated ventricle. When we selected a patient, clinical findings with a high level of possibility of ETV or endoscopic septostomy success results were considered. To perform ETV, we looked at the image and particularly considered the third ventricular floor bowing. Seventeen out of 25 patients had third ventricular floor bowing. Eight of them failed ETV, with a success rate of 52.9%, and one in eight patients without third ventricular floor bowing failed ETV. This result seemed to show ETV to be more effective in patients without third ventricular floor bowing. It did not show a statistically significant relationship. This may be because the turgor of a ventricle decreases after a long duration of shunt, and despite having obstructive hydrocephalus, the third ventricular floor bowing may not have been observed. Although we performed ETV on those who were deemed likely to succeed, sometimes we failed and had to perform shunt revision. Kim et al. [21] explained a possible mechanism of shunt dependency as ‘chronic idling’. After long-term shunting, CSF absorption regresses and needs to be shunted. Even if a hole is made successfully, it can fail due to shunt dependency; therefore, the patient and guardian need a sufficient explanation about the possible problems in advance.
Three of seven patients had unilateral hydrocephalus at the time of the first shunt operation. All of their symptoms improved through septostomy when malfunction occurred. Therefore, performing endoscopic septostomy is reasonable for patients who initially have unilateral hydrocephalus at the first shunt insertion.
Shunting can cause hardware problems, malfunction, infection, and overdrainage. Therefore, the endoscopic procedure should be considered at the time of shunt problems occurring before the revision of the shunt. Our study demonstrates that endoscopic procedures in the treatment of hydrocephalus or isolated unilateral hydrocephalus can be effective and safe when problems arise in patients who have previously undergone a shunt operation. We hope that through our experience and research, other clinicians will be able to make more informed decisions in treating patients with hydrocephalus who have VP shunt malfunction.
Notes
Author contributions
Conceptualization : SKK, KHK; Data curation : KHK, JYL, JHP, SKK; Formal analysis : KHK; Funding acquisition : SKK; Methodology : KHK, SKK; Project administration : SKK; Visualization : KHK, YS; Writing - original draft : KHK; Writing - review & editing : KHK, SKK, JYL, JHP, EJK
References
1. Albanese V, Tomasello F, Sampaolo S. Multiloculated hydrocephalus in infants. Neurosurgery. 8:641–646. 1981.
2. Aldana PR, Kestle JR, Brockmeyer DL, Walker ML. Results of endoscopic septal fenestration in the treatment of isolated ventricular hydrocephalus. Pediatr Neurosurg. 38:286–294. 2003.
3. Baldauf J, Fritsch MJ, Oertel J, Gaab MR, Schröder H. Value of endoscopic third ventriculostomy instead of shunt revision. Minim Invasive Neurosurg. 53:159–163. 2010.
4. Baskin JJ, Manwaring KH, Rekate HL. Ventricular shunt removal: the ultimate treatment of the slit ventricle syndrome. J Neurosurg. 88:478–484. 1998.
5. Bilginer B, Oguz KK, Akalan N. Endoscopic third ventriculostomy for malfunction in previously shunted infants. Childs Nerv Syst. 25:683–688. 2009.
6. Boschert J, Hellwig D, Krauss JK. Endoscopic third ventriculostomy for shunt dysfunction in occlusive hydrocephalus: long-term follow up and review. J Neurosurg. 98:1032–1039. 2003.
7. Brockmeyer D, Abtin K, Carey L, Walker ML. Endoscopic third ventriculostomy: an outcome analysis. Pediatr Neurosurg. 28:236–240. 1998.
8. Buxton N, Macarthur D, Robertson I, Punt J. Neuroendoscopic third ventriculostomy for failed shunts. Surg Neurol. 60:201–203. discussion 203-204. 2003.
9. Chan DYC, Tsang ACO, Ho WWS, Cheng KKF, Li LF, Tsang FCP, et al. Emergency endoscopic third ventriculostomy for blocked shunts? Univariate and multivariate analysis of independent predictors for failure. J Neurosurg. 131:1–7. 2018.
10. Cinalli G, Salazar C, Mallucci C, Yada JZ, Zerah M, Sainte-Rose C. The role of endoscopic third ventriculostomy in the management of shunt malfunction. Neurosurgery. 43:1323–1327. discussion 1327-1329. 1998.
11. Deopujari CE, Karmarkar VS, Shaikh ST. Endoscopic third ventriculostomy: success and failure. J Korean Neurosurg Soc. 60:306–314. 2017.
12. Dlouhy BJ, Capuano AW, Madhavan K, Torner JC, Greenlee JD. Preoperative third ventricular bowing as a predictor of endoscopic third ventriculostomy success. J Neurosurg Pediatr. 9:182–190. 2012.
13. Duru S, Peiro JL, Oria M, Aydin E, Subasi C, Tuncer C, et al. Successful endoscopic third ventriculostomy in children depends on age and etiology of hydrocephalus: outcome analysis in 51 pediatric patients. Childs Nerv Syst. 34:1521–1528. 2018.
14. Eller TW, Pasternak JF. Isolated ventricles following intraventricular hemorrhage. J Neurosurg. 62:357–362. 1985.
15. Hader WJ, Walker RL, Myles ST, Hamilton M. Complications of endoscopic third ventriculostomy in previously shunted patients. Neurosurgery. 63(1 Suppl 1):ONS168–ONS174. discussion ONS174-ONS175. 2008.
16. Hamada H, Hayashi N, Kurimoto M, Umemura K, Hirashima Y, Endo S. Neuroendoscopic septostomy for isolated lateral ventricle. Neurol Med Chir (Tokyo). 43:582–587. discussion 588. 2003.
17. Hopf NJ, Grunert P, Fries G, Resch KD, Perneczky A. Endoscopic third ventriculostomy: outcome analysis of 100 consecutive procedures. Neurosurgery. 44:795–804. discussion 804-806. 1999.
18. Jamjoom AB, Mohammed AA, al-Boukai A, Jamjoom ZA, Rahman N, Jamjoom HT. Multiloculated hydrocephalus related to cerebrospinal fluid shunt infection. Acta Neurochir (Wien). 138:714–719. 1996.
19. Jenkinson MD, Hayhurst C, Al-Jumaily M, Kandasamy J, Clark S, Mallucci CL. The role of endoscopic third ventriculostomy in adult patients with hydrocephalus. J Neurosurg. 110:861–866. 2009.
20. Kalsbeck JE, DeSousa AL, Kleiman MB, Goodman JM, Franken EA. Compartmentalization of the cerebral ventricles as a sequela of neonatal meningitis. J Neurosurg. 52:547–552. 1980.
21. Kim SK, Cho BK, Chung YN, Kim HS, Wang KC. Shunt dependency in shunted arachnoid cyst: a reason to avoid shunting. Pediatr Neurosurg. 37:178–185. 2002.
22. Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S, et al. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr. 155:254–259.e1. 2009.
23. Lazareff JA, Peacock W, Holly L, Ver Halen J, Wong A, Olmstead C. Multiple shunt failures: an analysis of relevant factors. Childs Nerv Syst. 14:271–275. 1998.
24. Lee SH, Kong DS, Seol HJ, Shin HJ. Endoscopic third ventriculostomy in patients with shunt malfunction. J Korean Neurosurg Soc. 49:217–221. 2011.
25. Marton E, Feletti A, Basaldella L, Longatti P. Endoscopic third ventriculostomy in previously shunted children: a retrospective study. Childs Nerv Syst. 26:937–943. 2010.
26. Melikian A, Korshunov A. Endoscopic third ventriculostomy in patients with malfunctioning CSF-shunt. World Neurosurg. 74:532–537. 2010.
27. Neils DM, Wang H, Lin J. Endoscopic third ventriculostomy for shunt malfunction: what to do with the shunt? Surg Neurol Int. 4:3. 2013.
28. Nida TY, Haines SJ. Multiloculated hydrocephalus: craniotomy and fenestration of intraventricular septations. J Neurosurg. 78:70–76. 1993.
29. O'Brien DF, Javadpour M, Collins DR, Spennato P, Mallucci CL. Endoscopic third ventriculostomy: an outcome analysis of primary cases and procedures performed after ventriculoperitoneal shunt malfunction. J Neurosurg. 103(5 Suppl):393–400. 2005.
30. Oertel JM, Schroeder HW, Gaab MR. Endoscopic stomy of the septum pellucidum: indications, technique, and results. Neurosurgery. 64:482–491. discussion 491-493. 2009.
31. Oi S, Hidaka M, Honda Y, Togo K, Shinoda M, Shimoda M, et al. Neuroendoscopic surgery for specific forms of hydrocephalus. Childs Nerv Syst. 15:56–68. 1999.
32. Sainte-Rose C, Piatt JH, Renier D, Pierre-Kahn A, Hirsch JF, Hoffman HJ, et al. Mechanical complications in shunts. Pediatr Neurosurg. 17:2–9. 1991.
33. Salmon JH. Isolated unilateral hydrocephalus following ventriculoatrial shunt. J Neurosurg. 32:219–226. 1970.
34. Shaikh S, Deopujari CE, Karmarkar V, Muley K, Mohanty C. Role of secondary endoscopic third ventriculostomy in children: review of an institutional experience. Pediatr Neurosurg. 54:188–195. 2019.
35. Siomin V, Cinalli G, Grotenhuis A, Golash A, Oi S, Kothbauer K, et al. Endoscopic third ventriculostomy in patients with cerebrospinal fluid infection and/or hemorrhage. J Neurosurg. 97:519–524. 2002.
36. Siomin V, Weiner H, Wisoff J, Cinalli G, Pierre-Kahn A, Saint-Rose C, et al. Repeat endoscopic third ventriculostomy: is it worth trying? Childs Nerv Syst. 17:551–555. 2001.
37. Spennato P, O'Brien DF, Fraher JP, Mallucci CL. Bilateral abducent and facial nerve palsies following fourth ventricle shunting: two case reports. Childs Nerv Syst. 21:309–316. 2005.
38. Teo C, Jones R. Management of hydrocephalus by endoscopic third ventriculostomy in patients with myelomeningocele. Pediatr Neurosurg. 25:57–63. discussion 63. 1996.
39. Waqar M, Ellenbogen JR, Mallucci C. Endoscopic third ventriculostomy for shunt malfunction in children: a review. J Clin Neurosci. 51:6–11. 2018.
40. Zhao R, Shi W, Yang H, Li H. Endoscopic third ventriculostomy instead of shunt revision in children younger than 3 years of age. World Neurosurg. 88:92–96. 2016.
Fig. 1.
Illustrative case of a patient who underwent endoscopic third ventriculostomy. A 23-year-old male who was diagnosed with germinoma in the pineal gland. Mid-sagittal magnetic resonance imaging shows third ventricular floor bowing. Reference line (blue) is drawn from the optic chiasm to the highest point of the midbrain. The arrow (red) indicates the distance between the reference line and the tuber cinereum.
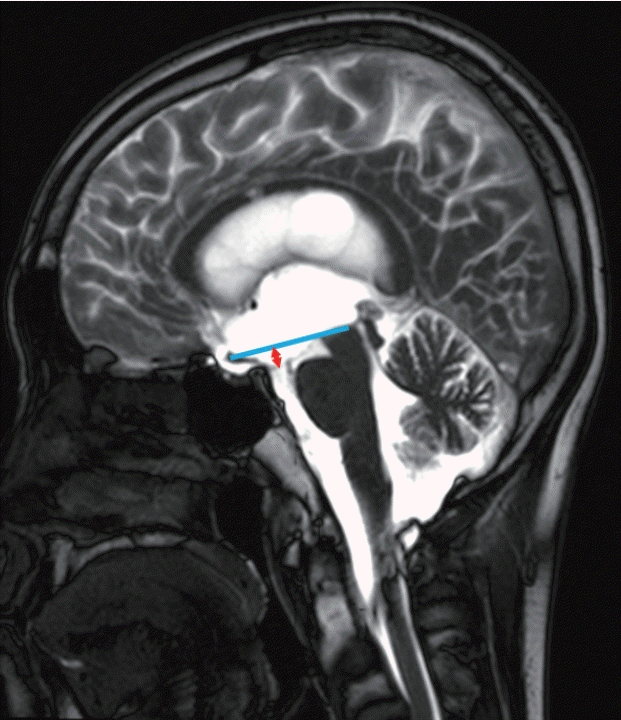
Fig. 2.
Illustrative case of a patient who underwent endoscopic third ventriculostomy. A 15-year-old female who presented with preterm intraventricular
hemorrhage. (A) Magnetic resonance imaging (MRI) shows no third ventricular floor bowing (asterisk). However, sagittal phase-contrast cine MR in diastole
(B) and systole (C) show decreased aqueductal cerebrospinal fluid (CSF) flow, but patent CSF flow passes through premedullary and cerebellomedullary
cistern. (D) Diminished CSF flow velocity at the aqueduct of Sylvius level is measured by phase-contrast MRI.
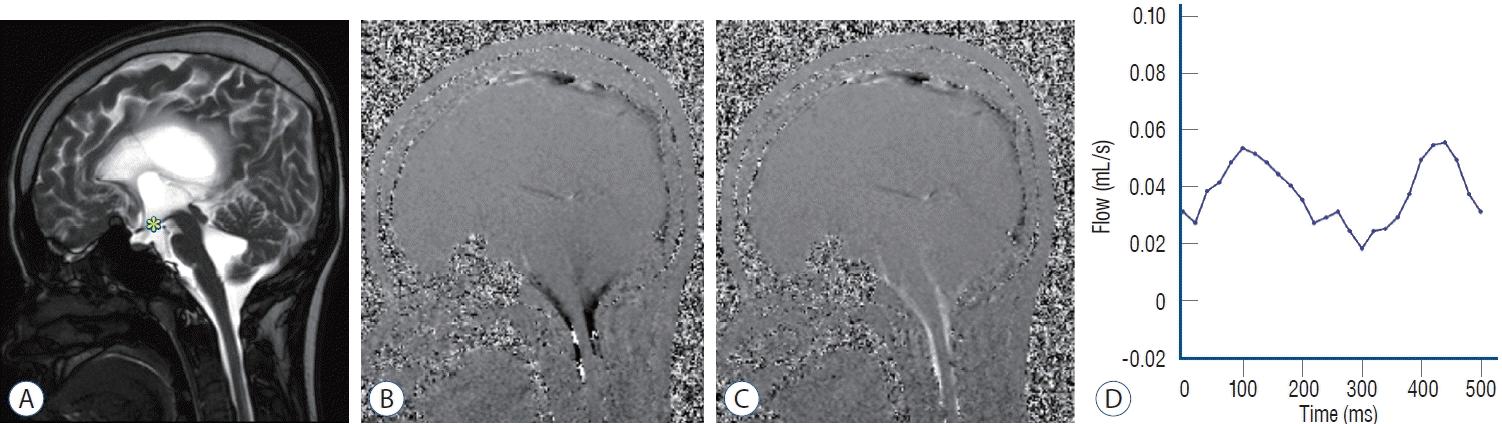
Fig. 3.
Kaplan-Meier curves showing overall survival. A : Patients with endoscopic third ventriculostomy for shunt malfunction. B : Patients with septostomy for isolated unilateral hydrocephalus.
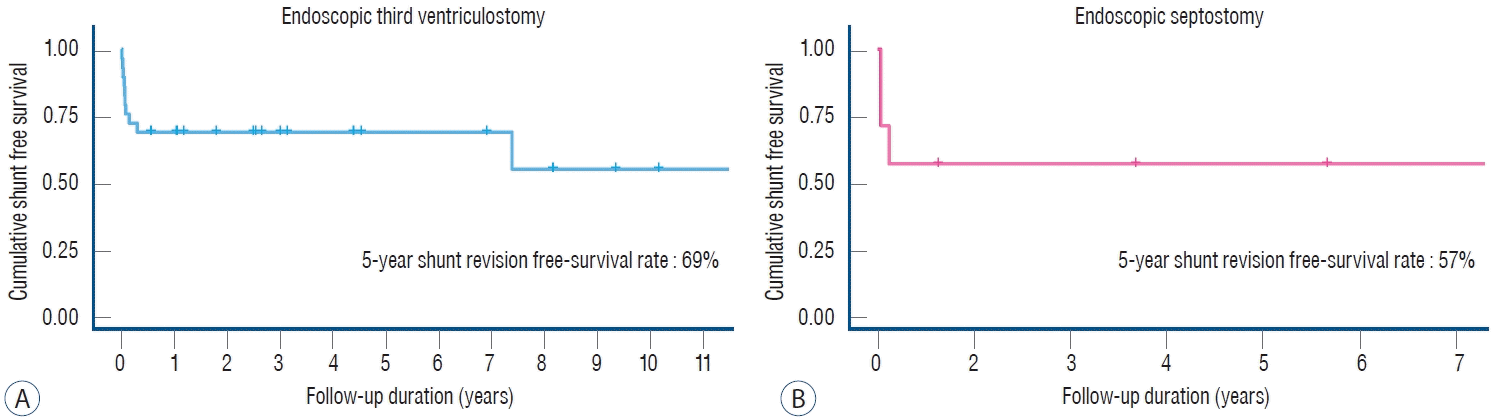
Table 1.
Characteristics of patients who were treated with ETV
Table 2.
Characteristics of patients who were treated with endoscopic septostomy
| Patient | Sex | Age* | Age† | Etiology of HC | Unilateral HC at shunt operation | Outcome |
|---|---|---|---|---|---|---|
| 1 | F | 12 years | 12 years | Postoperative (Rt. F pilocytic astrocytoma) | Yes | Success |
| 2 | F | 5 months | 18 months | Postinfectious | No | Success |
| 3 | F | 8 years | 29 years | Congenital (porencephalic cyst) | No | Failure |
| 4 | M | 6 years | 7 years | Postoperative (epilepsy) | No | Failure |
| 5 | F | 3 months | 26 months | Posthemorrhagic | Yes | Success |
| 6 | M | 5 months | 6 months | Postoperative (intraventricular immature teratoma) | No | Failure |
| 7 | M | 2 months | 27 years | Congenital (porencephalic cyst) | Yes | Success |
Table 3.
Univariate analysis of various prognostic factors for endoscopic third ventriculostomy failure
| Parameter |
Univariate analysis |
||
|---|---|---|---|
| OR* | 95% CI | p-value | |
| Age | 0.99 | 0.99 to 1.00 | 0.15 |
| Diagnosis | |||
| Congenital aqueductal stenosis | 3.56 | 0.61 to 20.81 | 0.16 |
| Tumor-related | 0.00 | 0.00 to Inf | 0.99 |
| Posthemorrhagic | 3.75 | 0.71 to 19.71 | 0.12 |
| Postinfectious | 0.59 | 0.05 to 6.57 | 0.67 |
| Reason for ETV | |||
| Malfunction | 0.50 | 0.03 to 8.95 | 0.63 |
| Infection | 2.00 | 0.11 to 35.81 | |
| Shunt duration | 0.99 | 0.98 to 1.00 | 0.07 |
| ETVSS | 0.98 | 0.90 to 1.06 | 0.57 |
Table 4.
Univariate analysis of various prognostic factors for endoscopic septostomy
| Parameter |
Univariate analysis |
||
|---|---|---|---|
| OR* | 95% CI | p-value | |
| Age | 1.00 | 0.99 to 1.01 | 0.87 |
| Diagnosis | |||
| Congenital porencephaly | 1.50 | 0.06 to 40.63 | 0.81 |
| Postoperative (2 tumors, 1 epilepsy) | 6.00 | 0.22 to 162.53 | 0.29 |
| Posthemorrhagic | 0.00 | 0.00 to Inf | 1.00 |
| Postinfectious | 0.00 | 0.00 to Inf | 1.00 |
| Unilateral HC at the shunt insertion | 0.17 | 0.01 to 4.51 | 0.28 |
| Shunt duration | 1.00 | 0.99 to 1.01 | 0.97 |
Table 5.
ETVSS for ETV patients
ETVSS is based on age, etiology of hydrocephalus, and presence of a previous shunt [22]. All patients in this study underwent shunt surgery, so the “previous shunt” scores were zero. ETVSS : ETV success score, ETV : endoscopic third ventriculostomy
Table 6.
Secondary endoscopic third ventriculostomy outcome data of published literature
| Study | No. of patients | ETV outcome (%shunt-free) |
|---|---|---|
| Baldauf et al [3]. | 30 | 60 |
| Baskin et al [4]. | 15 | 66 |
| Bilginer et al [5]. | 45 | 80 |
| Boschert et al [6]. | 17 | 82 |
| Brockmeyer et al [7]. | 36 | 42 |
| Buxton et al [8]. | 88 | 52 |
| Chan et al [9]. | 31 | 65 |
| Cinalli et al [10]. | 30 | 77 |
| Duru et al [13]. | 51 | 70 |
| Hader et al [15]. | 45 | 80 |
| Hopf et al [17]. | 25 | 84 |
| Jenkinson et al [19]. | 61 | 67 |
| Lee et al [24]. | 19 | 68 |
| Marton et al [25]. | 22 | 64 |
| Melikian and Korshunov [26] | 60 | 72 |
| Neils et al [27]. | 20 | 70 |
| O'Brien et al [29]. | 63 | 70 |
| Shaikh et al [34]. | 40 | 74 |
| Siomin et al [36]. | 20 | 65 |
| Teo and Jones [38] | 55 | 84 |
| Zhao et al [40]. | 37 | 60 |
| Present study | 29 | 66 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download