Inflammatory bowel disease (IBD) is an incurable condition characterized by immune dysregulation that primarily involves the gastrointestinal tract, phenotypically classified into Crohn’s disease (CD) and ulcerative colitis (UC) [
1]. Patients with IBD are subject to life-long multiple tests to monitor their disease progression. Recent survey of New Zealand parents of children with IBD reported that they preferred any tests less invasive than colonoscopy and preferred urine over a fecal test if given the choice [
2]. Based on this rationale, this study evaluated 2 urinary biomarkers that potentially detect different aspects of IBD: urinary chitinase 3-like 1 (CHI3L1) as an inflammatory marker [
3] and intestinal fatty-acid binding protein (I-FABP) as an epithelial barrier marker [
4]. Urinary CHI3L1 is yet to be explored in the setting of IBD, whilst a recent study found urinary I-FABP to be a potential disease activity marker in adults with CD [
5]. This study evaluated the role of these 2 urinary markers in children with IBD.
Children between 5 and 16 years of age were prospectively recruited from Christchurch Hospital, New Zealand between September 2018 and July 2020. The study was approved by the New Zealand Southern Health and Disability Ethics Committees (reference 18/STH/136) and performed in accordance with the principles of the Declaration of Helsinki. Informed consent and assent were obtained from parents and children, respectively. Participants were categorized into 5 groups: active IBD, inactive IBD, non-IBD, healthy sibling (HS), and celiac disease (CeD). Patients with IBD were diagnosed based on the revised Porto criteria [
1]. The active IBD group included those with newly diagnosed untreated IBD or those with relapsed disease requiring treatment to reinduce remission. The inactive IBD group included participants with known IBD who were in clinical remission based on clinical indexes (Pediatric Crohn’s Disease Activity Index [PCDAI] < 10 for CD or Pediatric Ulcerative Colitis Activity Index [PUCAI] < 10 for UC) and had no alteration to their IBD medications 3 months prior to enrolment. Children who underwent investigations for gastrointestinal symptoms and in whom IBD was excluded were categorized as the non-IBD group. Siblings of children with known IBD who were reported to be healthy were enrolled into the HS group. Children with newly diagnosed untreated CeD (based on any positive celiac serologies and histological modified Marsh-Oberhuber classification ≥ 2) were included as a disease control group.
Baseline demographics including clinical (PCDAI and PUCAI) and endoscopic disease activity (Simple Endoscopic Score for CD [SES-CD] and Mayo endoscopic score) were recorded. All disease groups provided baseline blood and urine samples, whilst HS group provided a urine sample only. Urine samples were collected and stored at 4°C within 24 hours of participant’s clinic appointment. Received samples were immediately aliquoted and stored at –80°C. Samples for analysis were thawed, centrifuged at 1,000 ×g for 20 minutes and the supernatant collected. An aliquot of each supernatant was forwarded to a reference laboratory for measurement of creatinine (Cr) levels as a means to standardize urine concentrations. Commercial ELISA kits designed to detect human CHI3L1 (R&D Systems, Minneapolis, MN, USA) and I-FABP (Hycult® Biotech, Uden, the Netherlands) were used to determine levels of these biomarkers (in duplicate) in each urine supernatant. Each assay was performed according to the respective manufacturers’ instructions.
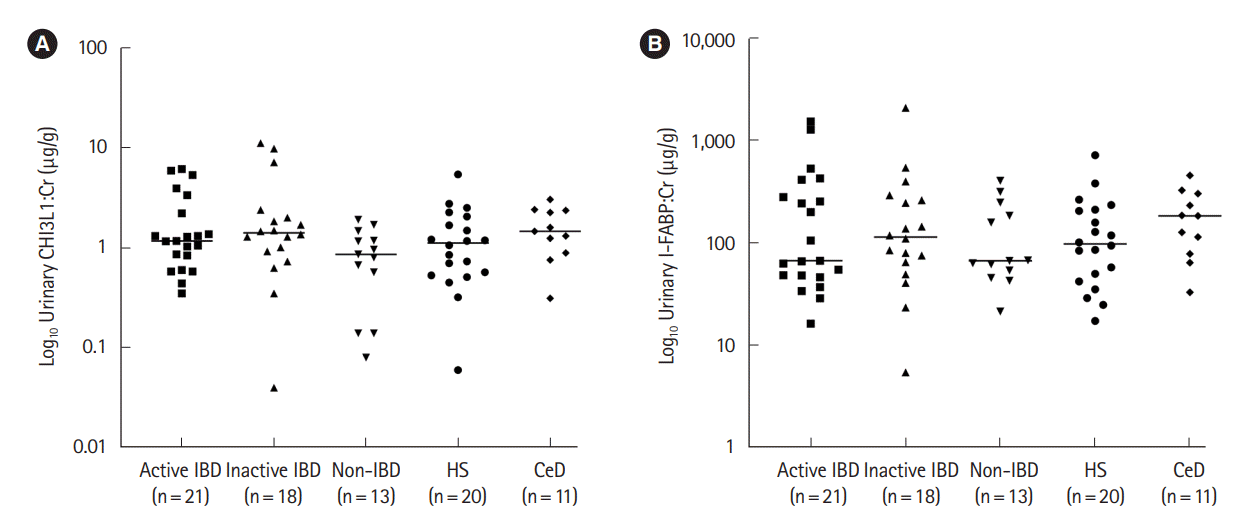
Ninety-five children were recruited with 12 patients excluded (11 withdrew and 1 had concurrent IBD and CeD), leaving 83 children for final analysis: 21 participants in the active IBD (15 new CD, 4 relapsed CD, 2 new UC), 18 in the inactive IBD, 13 in the non-IBD, 20 in the HS and another 11 in the CeD group (
Table 1). Median (interquartile range) urinary CHI3L1 and I-FABP standardized to urine Cr (CHI3L1:Cr and I-FABP:Cr, respectively) levels (
Fig. 1) were not different between study groups (Kruskal-Wallis test,
P>0.05 for each). When urinary CHI3L1:Cr and I-FABP:Cr levels were compared with blood results (albumin, platelet count, C-reactive protein, and erythrocyte sedimentation rate) from the disease groups, there were no differences in either biomarker (Mann-Whitney test,
P>0.05 for each test). There were no correlations between urinary biomarkers (CHI3L1:Cr and I-FABP:Cr) and PCDAI and SES-CD: PCDAI (n = 35); Spearman’s
r=–0.08 for urinary CHI3L1:Cr and I-FABP: Cr,
P>0.05 and SES-CD (n = 17); Spearman’s
r=–0.21 for urinary CHI3L1:Cr and
r=–0.29 for urinary I-FABP:Cr,
P>0.05. Analysis between urinary biomarkers and PUCAI and Mayo endoscopic score was not performed due to the small sample size of patients with UC.
This study evaluated urinary CHI3L1 and I-FABP biomarkers levels as biomarkers of inflammation and epithelial barrier integrity, respectively, with the idea of potentially detecting different aspects of IBD. CHI3L1, a glycoprotein that binds to heparin, chitin, hyaluronan and collagen without any catalytic activity is elevated in several inflammatory conditions, including IBD [
3]. Three studies have shown higher CHI3L1 levels in patients with active IBD (based on clinical indexes) than in healthy controls [
6-
8]. Although no published study has yet evaluated urinary CHI3L1 in the setting of IBD, urinary CHI3L1 has been explored in the identification of urinary tract infection in young children, especially those with negative urine nitrite on dipstick assessment [
9]. Investigators noted that urinary CHI3L1 levels correlated positively with serum white blood cell count and C-reactive protein levels, suggesting that elevation of urinary CHI3L1 mirrored systemic inflammatory changes. In the present study, urinary CHI3L1 levels did not differ between patients with normal and those with abnormal blood inflammatory markers.
I-FABP is 1 of 9 known cytoplasmic proteins that primarily regulate the intracellular transportation of long-chain fatty acids with more prominent expression found in jejunal villi than in colonocytes [
4]. Following enterocyte damage, I-FABP is released into the circulation and cleared rapidly by the kidneys (plasma half-life of 11 minutes), similar to other fatty-acid binding proteins [
4]. A recent study found that urinary I-FABP levels fall in young adults with active CD following 8 weeks of exclusive enteral nutrition, suggesting that it may be a sensitive indicator of disease activity [
5]. In another study, urinary and plasma I-FABP were assessed among 138 children with IBD and CeD (47 with CD, 11 with UC, 12 with newly diagnosed CeD, 40 with established CeD and 28 without IBD or CeD), plasma I-FABP levels were significantly higher in the children with newly diagnosed CeD and those with CD only when compared to those without IBD or CeD [
10]. Otherwise, urinary and plasma I-FABP levels did not differ between the rest of the groups, which is consistent with the results in the current study. The present study was limited by small sample size with predominately patients with CD of mild disease severity and other available biomarkers, such as fecal calprotectin, were not available for comparison. Paired serum and urinary biomarker levels would be useful in future studies.
In conclusion, urinary CHI3L1 and I-FABP were unable to differentiate children with active IBD from those without IBD, and did not correlate with PCDAI or SES-CD. These data suggest that measurement of either biomarker in urine is unlikely to have any diagnostic or monitoring role in pediatric IBD.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download