Abstract
Background/Aims
Methods
Results
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contribution
Conceptualization: Koutroubakis IE. Data curation: all authors. Formal analysis: Mantaka A. Investigation: Mantaka A, Galanakis N, Tsetis D. Methodology: all authors. Project administration: Mantaka A, Galanakis N. Supervision: Tsetis D, Koutroubakis IE. Writing - original draft: Mantaka A. Writing - review & editing: Galanakis N, Tsetis D, Koutroubakis IE. Approval of final manuscript: all authors.
REFERENCES
Fig. 1.
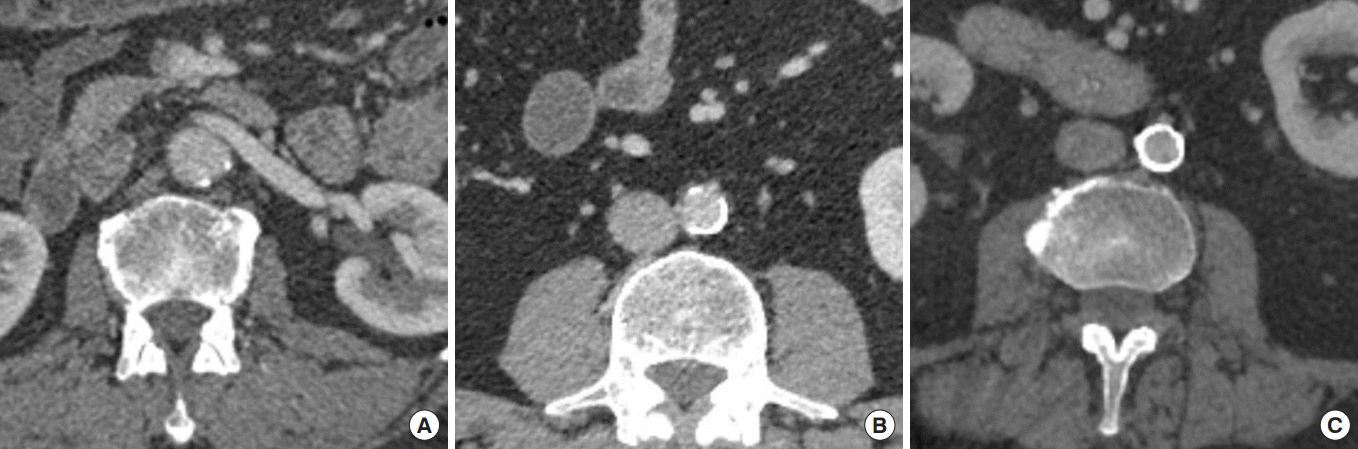
Table 1.
| Characteristics | IBD patients (n = 98) | IBD with CVD (n = 24) | IBD without CVD (n = 74) | P-value | OR (95% CI) |
|---|---|---|---|---|---|
| Age at diagnosis (yr) | 44.78 ± 15.48 | 54.42 ± 12.65 | 41.65 ± 15.08 | < 0.001 | 3.42 (5.99–19.55) |
| Age at study entry (yr) | 58.75 ± 14.45 | 68.17 ± 10.39 | 55.40 ± 14.20 | < 0.001 | 3.14 (6.53–19.01) |
| IBD duration at CTE/CT (yr) | 11.67 ± 10.13 | 11.12 ± 10.56 | 11.85 ± 10.05 | NS | |
| IBD duration (yr) | 14.04 ± 10.40 | 13.66 ± 10.60 | 14.16 ± 10.41 | NS | |
| Female sex | 29 (29.6) | 4 (13.8) | 25 (86.2) | < 0.001 | |
| Body mass index (kg/m2) | 27.25 ± 4.93 | 31.62 ± 27.41 | 29.17 ± 25.44 | 0.006 | 1.22 (1.02–5.89) |
| Smoking status (n = 95) | < 0.001 | ||||
| Ever smokers | 75 (79.0) | 20 (87.0) | 55 (76.4) | ||
| Never smokers | 20 (21.1) | 3 (13.0) | 17 (23.6) | ||
| Diagnosis | < 0.001 | ||||
| UC | 30 (30.6) | 11 (45.8) | 19 (25.7) | ||
| CD | 68 (69.4) | 13 (54.2) | 55 (74.3) | ||
| CD Montreal classification age | < 0.001 | ||||
| A1 | 1 (1.0) | 1 (4.2) | 0 | ||
| A2 | 36 (36.7) | 4 (16.7) | 32 (43.2) | ||
| A3 | 61 (62.3) | 19 (79.2) | 42 (56.8) | ||
| Location (n = 68) | < 0.001 | ||||
| L1 | 30 (44.1) | 6 (46.1) | 24 (43.6) | ||
| L2 | 12 (17.7) | 3 (23.1) | 9 (16.4) | ||
| L3 | 26 (38.2) | 4 (30.8) | 22 (40.0) | ||
| Behavior (n = 68) | < 0.001 | ||||
| B1 | 32 (47.1) | 8 (61.5) | 24 (43.6) | ||
| B2 | 29 (42.6) | 3 (23.1) | 26 (47.3) | ||
| B3 | 7 (10.3) | 2 (15.4) | 5 (9.0) | ||
| Perianal disease (n = 69)a | < 0.001 | ||||
| Perianal disease | 15 (21.7) | 5 (35.7) | 10 (18.2) | ||
| No perianal disease | 54 (78.3) | 9 (64.3) | 45 (81.8) | ||
| UC Montreal classification extent (n = 30) | < 0.001 | ||||
| E1 | 2 (66.7) | 2 (18.2) | 0 | ||
| E2 | 13 (43.3) | 6 (54.5) | 7 (36.8) | ||
| E3 | 15 (50.0) | 3 (27.3) | 12 (63.2) | ||
| Musculoskeletal extraintestinal manifestations | 35 (35.7) | 12 (50.0) | 23 (31.1) | < 0.001 | |
| IBD related surgery | 18 (18.4) | 3 (12.5) | 15 (20.3) | < 0.001 | |
| Endoscopic lesions: severe lesions (n = 97) | 28 (28.9) | 3 (12.5) | 25 (34.3) | < 0.001 | |
| CTE/CT findings | |||||
| Disease extend: extensive disease (n = 96) | 41 (42.7) | 7 (29.2) | 34 (47.2) | < 0.001 | |
| Inflammatory disease: active (n = 68) | 56 (57.1) | 10 (41.7) | 46 (61.6) | < 0.001 | |
| Stricturing diseaseb | 28 (28.6) | 3 (12.5) | 25 (33.8) | < 0.001 |
Table 2.
Table 3.
| Variable | None-mild atherosclerotic lesions (AAC) | Moderate-severe atherosclerotic lesions (AAC) | P-value |
|---|---|---|---|
| IBD patient (n = 98) | 63 (64.3) | 35 (35.7) | 0.544 |
| Control (n = 98) | 68 (69.4) | 30 (30.6) | |
| IBD with CVD (n = 24) | 8 (33.3) | 16 (66.7) | 1.000 |
| Control with CVD (n = 24) | 9 (37.5) | 15 (62.5) | |
| IBD without CVD (n = 74) | 55 (74.3) | 19 (25.7) | 0.558 |
| Control without CVD (n = 74) | 59 (79.7) | 15 (20.3) | |
| IBD with CVD (n = 24) | 8 (33.3) | 16 (66.7) | 0.001 |
| IBD without CVD (n = 74) | 55 (74.3) | 19 (25.7) | |
| UC | 13 (43.3) | 17 (56.7) | 0.010 |
| CD | 50 (73.5) | 18 (26.5) | |
| Age at diagnosis (yr) | 40.25 ± 14.52 | 52.91 ± 13.89 | < 0.001 |
| IBD duration at CTE/CT (yr) | 10.68 ± 9.21 | 13.47 ± 11.52 | 0.193 |
| Sex | |||
| Female, IBD | 20 (69.0) | 9 (31.0) | 0.773 |
| Female, control | 21 (72.4) | 8 (27.6) | |
| Male, IBD | 43 (62.3) | 26 (37.7) | 0.475 |
| Male, control | 47 (68.1) | 22 (31.9) | |
| CD Montreal location (n = 68) | 0.370 | ||
| L1 | 21 (70.0) | 9 (30.0) | |
| L2 | 8 (66.7) | 4 (33.3) | |
| L3 | 21 (80.8) | 5 (19.2) | |
| CD behavior (n = 68) | 0.396 | ||
| B1 | 22 (68.7) | 10 (31.3) | |
| B2 | 22 (75.9) | 7 (24.1) | |
| B3 | 6 (85.7) | 1 (14.3) | |
| Perianal disease (n = 69)a | 38 (70.4) | 16 (29.6) | 0.366 |
| IBD surgery | 15 (85.3) | 3 (16.7) | 0.123 |
| Anti-TNF-α agents | 43 (75.4) | 14 (24.6) | 0.016 |
| Lifetime steroids | 57 (73.1) | 21 (26.9) | 0.001 |
| Immunomodulator | 44 (67.7) | 21 (32.3) | 0.452 |
| Endoscopic lesions: severe lesions (n = 97) | 25 (89.3) | 3 (10.7) | 0.004 |
| CTE/CT findings | |||
| Disease extend: extensive disease (n = 96) | 31 (75.6) | 10 (24.4) | 0.060 |
| Inflammatory disease (n = 68) | 40 (81.6) | 9 (18.4) | 0.026 |
| Stricturing disease | 22 (78.6) | 6 (21.4) | 0.123 |
| Musculoskeletal extraintestinal manifestations | 20 (57.1) | 15 (42.9) | 0.401 |
| Body mass index (kg/m2) | 27.47 ± 4.80 | 26.82 ± 5.25 | 0.554 |
| Smoking history (n = 95) | |||
| Ever smokers | 45 (60.0) | 30 (40.0) | 0.097 |
| Never smokers | 17 (85.0) | 3 (15.0) | |
| Hypertension | 11 (33.3) | 22 (66.7) | < 0.001 |
| Diabetes mellitus | 5 (41.7) | 7 (58.3) | 0.155 |
| Dyslipidemia | 10 (35.7) | 18 (64.3) | 0.001 |
| Hemoglobin (g/dL) | 13.27 ± 1.67 | 13.43 ± 1.44 | 0.657 |
| C-reactive protein (mg/dL) | 2.26 ± 4.76 | 0.82 ± 1.40 | 0.100 |
| Glucose (mg/dL) | 105.33 ± 51.82 | 106.45 ± 22.52 | 0.909 |
| Cholesterol (mg/dL) | 187.04 ± 64.75 | 166.44 ± 33.44 | 0.129 |
| Albumin (mg/dL) | 3.98 ± 0.59 | 4.16 ± 0.45 | 0.182 |
Table 4.
Variables entered: age IBD diagnosis, IBD duration, IBD diagnosis, Montreal behavior, sex, smoking, body mass index, CTE findings (disease extent), anti-tumor necrosis factor α use, lifetime steroids, immunomodulators, hypertension, dyslipidemia, diabetes.
IBD, inflammatory bowel disease; aOR, adjusted odds ratio; CI, confidence interval; CTE, computed tomography enterography; CT, computed tomography.
Table 5.
| Variable | aOR (95% CI) | P-value |
|---|---|---|
| Extensive IBD | 25.661 (1.714–384.181) | 0.019 |
| Anti-TNF-α use | 0.023 (0.001–0.594) | 0.023 |
| Steroids lifetime | 18.287 (1.141–292.983) | 0.040 |
Variables: age at IBD diagnosis, Montreal behavior (B2 vs. B1, B3), computed tomography enterography findings (IBD extent), anti-TNF-α, steroids, sex, smoking, body mass index, hypertension, diabetes, dyslipidemia.
IBD, inflammatory bowel disease; aOR, adjusted odds ratio; CI, confidence interval; TNF, tumor necrosis factor.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download