Abstract
Notes
Funding Source
The work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES): Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPQ), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Pró-Reitoria de Pesquisa da UFMG (PRPq) and Specialization course in Pharmacology of Department of Pharmacology, ICB-UFMG.
Author Contribution
Conceptualization: Lima PA, Castor MGM. Data curation: Lima PA. Formal analysis: Castor MGM. Funding acquisition: Castor MGM. Investigation: Lima PA. Methodology: Lima PA, Berg BB, Castor MGM. Supervision: Castor MGM. Validation: Berg BB. Writing-original draft: Lima PA, Berg BB, Castor MGM. Writing-review & editing: Lima PA, Berg BB, Castor MGM. Approval of final manuscript: all authors.
REFERENCES
Fig. 1.
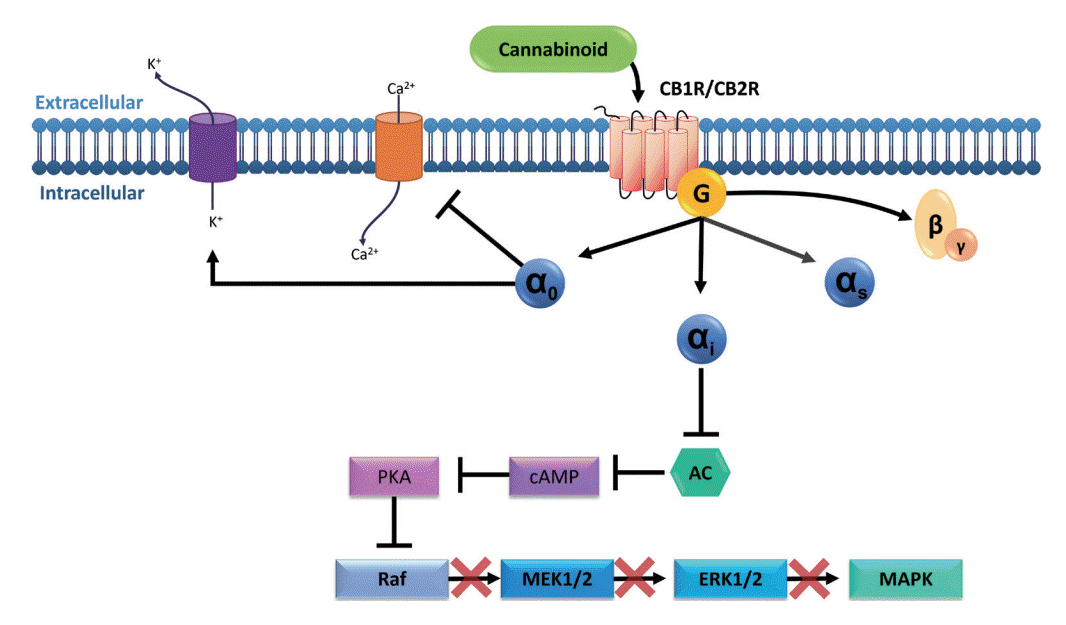
Fig. 2.
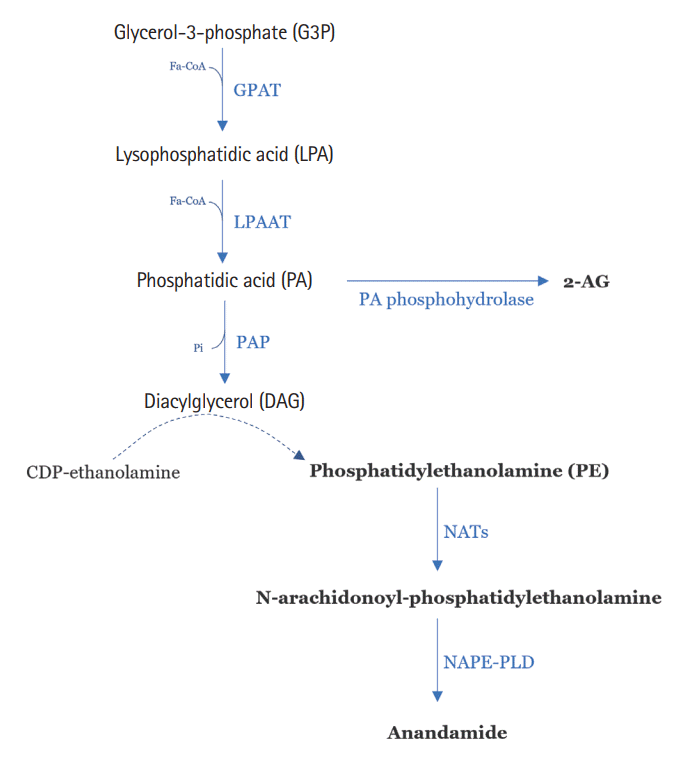
Fig. 3.
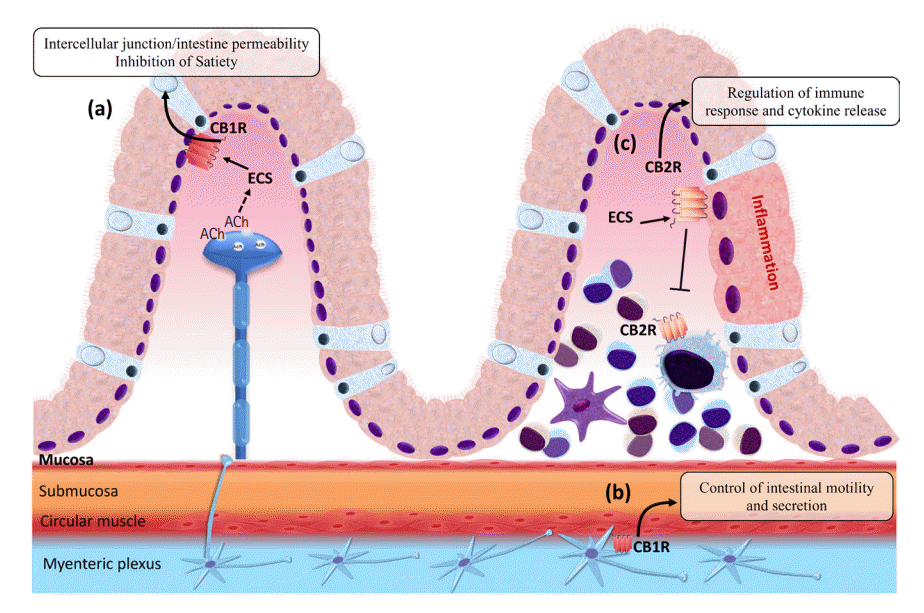
Fig. 4.
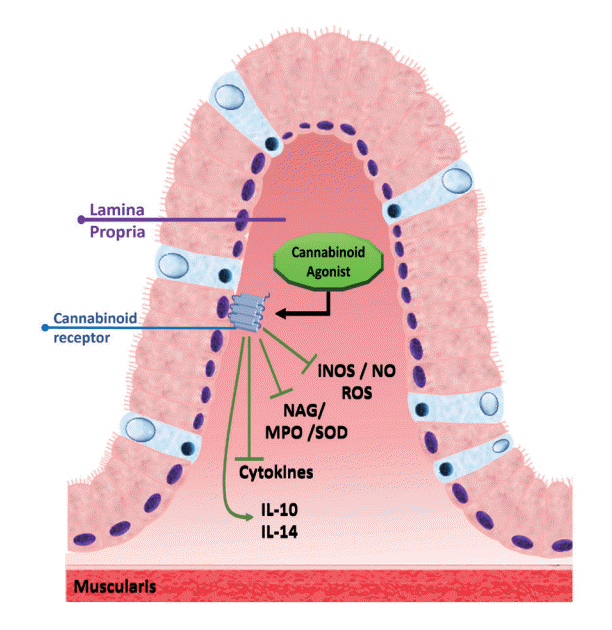
Fig. 5.
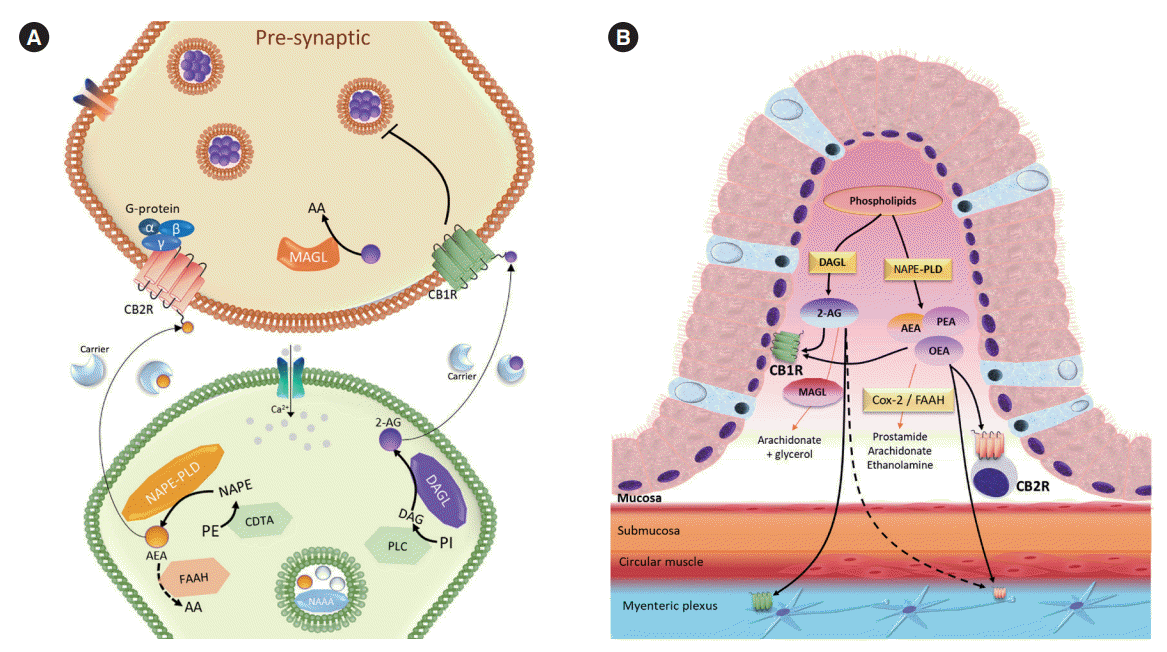
Table 1.
| Components of the ECS | Expression | Tissue analyzed | First author (year) |
|---|---|---|---|
| Receptor | |||
| CB1R | Increased | CHO-GLP-1R cells transduced with lentiviral of the CB1R isoforms | Kimball (2006) [102], Engel (2010) [84] |
| CB2R | Increased | C57BL/6j mice brain | Storr (2009) [85], Marquéz (2009) [96], Engel (2010) [84], Harvey (2013) [98], Ke (2016) [86] |
| Human brain tissues | |||
| C57BL/10J and the BTBR T+tF/J peripheral tissues and brain | |||
| Endocannabinoids binders | |||
| AEA | Increased | Colon sample from DNBS-treated mice | D'Argenio (2006) [97], Harvey (2013) [98], Sasso (2015) [93], Grill (2019) [100] |
| CD1 mice colon mucosa | |||
| ICR mice colon | |||
| 2-AG | Increased | ICR mice colon | Borrelli (2009) [90] |
| Synthesizing enzymes | |||
| NAPE-PLD | Increased | Human colonic endoscopic biopsies | Marquéz (2009) [96], Suárez (2012) [101] |
| Decreased | Human colonic endoscopic biopsies | Grill (2019) [100], Suárez (2012) [101] | |
| DAGL | Increased | Human colonic tissue | Marquéz (2009) [96] |
| Metabolizing enzymes | |||
| FAAH | Decreased | ICR mice colon | Storr (2008) [87], Borrelli (2009) [90], Sałaga (2014) [92], Tourteau (2014) [91], Sasso (2015) [93] |
| C57B1/6 colon samples | |||
| Rat brain homogenates | |||
| Increased | Human colonic tissue | Suárez (2012) [101] | |
| MAGL | Decreased | C57BL/6 mice colon or ileum | Alhouayek (2011) [95], Grill (2019) [94] |
| Increased | Human colonic tissue | Marquéz (2009) [96] |
ECS, endocannabinoid system; CB1R, cannabinoid receptor 1; CB2R, cannabinoid receptor 2; AEA, anandamide; DNBS, dinitrobenzene sulphonic acid; ICR, Institute of Cancer Research; 2-AG, 2-arachidonyl glycerol; NAPE-PLD, N-acyl phosphatidylethanolamine-specific phospholipase D; DAGL, diacylglycerol lipase; FAAH, fatty acid amide hydrolase; MAGL, monoacylglycerol lipase.
Table 2.
| Components of the endocannabinoid system | Expression | Tissue analyzed | First author (year) |
|---|---|---|---|
| Receptor | |||
| CB1R | Increased | Human colonic tissue | Stintzing (2011) [105] |
| CB2R | Increased | TNFΔARE/+ mice ileum | Leinwand (2017) [104] |
| Binders | |||
| AEA | Increased | TNFΔARE/+ mice ileum | Leinwand (2017) [104], Grill (2019) [100] |
| Human intestinal mucosal biopsy from patients with UC | |||
| Decreased | Intestinal mucosa biopsy from patients with CD | Di Sabatino (2011) [59] | |
| 2-AG | Increased | Human intestinal mucosa biopsy from patients with UC | Grill (2019) [100] |
| Synthesizing enzymes | |||
| NAPE-PLD | Increased | Human intestinal mucosa biopsy from patients with UC | Grill (2019) [100] |
| Decreased | Intestinal mucosa biopsy from patients with CD | Di Sabatino (2011) [59] | |
| DAGL | Increased | Human intestinal mucosa biopsy from patients with UC | Grill (2019) [100] |
| Metabolizing enzymes | |||
| FAAH | Increased | Human intestinal mucosal biopsy from patients with UC or CD | Di Sabatino (2011) [59], Grill (2019) [100] |
| MAGL | Increased | Human intestinal mucosal biopsy from patients with UC | Grill (2019) [100] |
ECS, endocannabinoid system; CD, Crohn's disease; CB1R, cannabinoid receptor 1; CB2R, cannabinoid receptor 2; TNFΔARE, tumor necrosis factor modified AUUUA-pentanucleotide rich elements; AEA, anandamide; UC, ulcerative colitis; 2-AG, 2-arachidonyl glycerol; NAPE-PLD, N-acyl phosphatidylethanolamine-specific phospholipase D; DAGL, diacylglycerol lipase; FAAH, fatty acid amide hydrolase; MAGL, monoacylglycerol lipase.
Table 3.
| Animal | Inflammation induction | Results after phytocannabinoid administration | First author (year) | |
|---|---|---|---|---|
| CBD–CBR agonist and FAAH inhibitor | ||||
| Male ICR mice | CO | - Hypermotility induced by CO was reduced. | Capasso (2008) [108] | |
| - The administration of rimonabant (CB1R antagonist) neutralized The action of CBD, however, The administration of SR144528 (CB2R antagonist) did not neutralize The effect of CBD. Indicating that CBD acts predominantly on CB1R receptors. | ||||
| - The effects of the joint administration of CBD, loperamide, and AA-5-HT were not additives to improve inflammation. | ||||
| - Inhibition of ACh-induced contractions in the ileum from mice treated with CO. | ||||
| Matured Sprague–Dawley rats and C57/BL mice | LPS | - Improvement of edema and infiltration of inflammatory cells. | Lin (2011) [109] | |
| - Prevention of increased levels of TNF-α and IL-6 in rats and mice. In rats, the action on TNF-α was greater and in mice, the action was greater on IL-6. | ||||
| - Normalization of ileum and colon contractions. | ||||
| Male ICR mice | DNBS | - Weight gain in mice. | Borrelli (2009) [90] | |
| - Reduced colon weight /colon length ratio. | ||||
| - Macroscopic damage, including edema, mild hyperemia, and adhesions of the small intestine were decreased. | ||||
| - iNOS expression was reduced. | ||||
| - Decrease in the synthesis of nitrite and NO stable metabolites. | ||||
| - IL-1β levels reduced. | ||||
| - IL-10 levels increased. | ||||
| - AEA and 2-AG levels normalized. | ||||
| - It was not cytotoxic to CACO-2 cells. | ||||
| - Antioxidant effect observed in CACO-2 cells. | ||||
| Human rectal cells | LPS+IFN-γ | - S100B protein expression was reduced. | De Filippis (2011) [110] | |
| - iNOS expression, NO levels, and stable metabolites were reduced. | ||||
| Male Swiss OF1 mice | LPS | - S100B protein expression was decreased. | ||
| - Decrease in mast cells in the intestinal tissue. | ||||
| - MAC3 in the intestine was reduced. | ||||
| - TNF-α was reduced. | ||||
| - Reduced immunoreactivity for caspase-3. | ||||
| Males CD1 mice | TNBS | - Improved colitis score. | Schicho (2012) [111] | |
| - Reduced MPO activity. | ||||
| - Less destruction of the epithelial lining. | ||||
| - Reduction in the thickness of the colon. | ||||
| - Decreased immunocyte infiltration. | ||||
| Humans with CD | - | - Absence of significant changes. | Naftali (2017) [112] | |
| Cannabis sativa extract rich in CBD–CBR agonist and FAAH inhibitor | ||||
| Male ICR mice | CO | - Reduction of intestinal motility. | Pagano (2016) [113] | |
| DNBS | - Reduced colon weight/colon length ratio. | |||
| - Reduced MPO activity. | ||||
| - Intestinal motility was reduced. | ||||
| Humans with mild to moderate UC | - | - Greater remission of the disease. | Irving (2018) [114] | |
| - Better quality of life. | ||||
| - Reduction in the Mayo total score. | ||||
| CBD and THC–CBR agonist and FAAH inhibitor | ||||
| Male Charles River Wistar rats | TNBS | - THC+CBD resulted in a reduction in weight gain. | Jamontt (2010) [115] | |
| - THC+CBD promoted a reduction in macroscopic damage. | ||||
| - THC+CBD reduced the MPO activity. | ||||
| - THC+CBD increased the range of low-frequency spontaneous contractions in the colon. | ||||
| - The substances improved the contraction response to carbachol. | ||||
| - Increased contractile and relaxing responses in the colon by EFS, with the administration of THC and its combination with CBD. | ||||
| THC–CBR agonist | ||||
| Humans with CD | - | - Greater remission of the disease. | Naftali (2013) [116] | |
| - Better quality of life. | ||||
| - Improves patients' appetite and sleep. | ||||
| CBN–CBR agonist | ||||
| Male ICR mice | CO | - Intestinal motility was reduced. | Izzo (2001) [117] | |
| - Improvement of inflammation. | ||||
| CBG–indirect activation of CBR | ||||
| Male ICR mice | DNBS | - Reduced colon weight/colon length ratio. | Borrelli (2013) [118] | |
| - Signs of colon damage (glands in regeneration, reduced edema, only superficial erosion) were reduced. | ||||
| - Ki-67 immunopositive cells in the lower half of the mucosa. | ||||
| - Intestinal permeability was reduced. | ||||
| - Reduced MPO activity. | ||||
| - Increased SOD activity. | ||||
| - iNOS expression reduced. | ||||
| - IL-1β and IFN-γ levels were decreased. | ||||
| - Increased levels of IL-10. | ||||
| Peritoneal macrophages | LPS | - Decreased nitrite synthesis. | ||
| - iNOS levels reduced. | ||||
| Ptk6 null colonic epithelial cells | Fenton reagent (H2O2+Fe2+) | - ROS synthesis reduced. | ||
| CBC–weak inhibitor from MAGL | ||||
| Male ICR mice | CO | - Intestinal motility was reduced. | Izzo (2012) [99] | |
| - Reduced ACh-induced contractions and EFS-induced contractions too. | ||||
| Peritoneal macrophages | LPS | - Reduced nitrite levels. | Romano (2013) [106] | |
| - Did not affect iNOS and COX-2 expression. | ||||
| - IFN-γ and IL-10 were reduced. | ||||
| - Did not decrease IL-1β levels. | ||||
| Male ICR mice | DNBS | - Reduced colon weight/colon length ratio. | ||
| - Decrease in intestinal permeability. | ||||
| - Reduced MPO activity. | ||||
| - Improvement in tissue damage. | ||||
| - Ki-67 immunoreactivity revealed the mitotic activity is restricted to one-half of the mucosa. | ||||
CBD, cannabidiol; CBR, cannabinoid receptor; FAAH, fatty acid amide hydrolase; ICR, Institute of Cancer Research; CO, croton oil; CB1R, cannabinoid receptor 1; CB2R, cannabinoid receptor 2; AA-5-HT, N-arachidonoyl-serotonin; ACh, acetylcholine; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor α; IL, interleukin; DNBS, dinitrobenzene sulphonic acid; iNOS, inducible nitric oxide synthase; NO, nitric oxide; AEA, anandamide; 2-AG, 2-arachidonyl glycerol; CACO-2 cells, human colon adenocarcinoma cells; IFN-γ, interferon γ; MAC3, macrophage differentiation antigen; TNBS, 2,4,6-trinitrobenzene sulfonic acid; MPO, myeloperoxidase; CD, Crohn's disease; UC, ulcerative colitis; THC, tetrahydrocannabinol; EFS, electrical field stimulation; SOD, superoxide dismutase; ROS, reactive oxygen species; MAGL, monoacylglycerol lipase; COX-2, cyclooxygenase-2.
Table 4.
| Synthetic cannabinoid | Animal | Inflammation induction | Results after administration of synthetic cannabinoids | First author (year) | |
|---|---|---|---|---|---|
| CB1R and CB2R agonists | |||||
| AM-841 | Males CD1 WT mice and knockout CB1R -/-, CB2R -/-, CB1/2R -/- | DSS | - Decrease of macroscopic and microscopic colonic damage. | Fichna (2014) [120] | |
| - Decreased immune cell infiltrate. | |||||
| - Reduced MPO activity. | |||||
| - Absence of an anti-inflammatory effect of AM-841 in knockout mice. | |||||
| Males CD1 mice | TNBS | - Decrease of macroscopic and microscopic damage. | |||
| - Reduced MPO activity. | |||||
| - No changes were seen in ulcer score or body weight. | |||||
| HU-210 | Female C57BL/6N WT mice, CB1R-deficient and FAAH-deficient | DNBS | - Mice with CB1R-deficient had the most pronounced inflammation. | Massa (2004) [61] | |
| - Decrease of macroscopic and microscopic damage. | |||||
| - Reduced MPO activity. | |||||
| C57BL/10J WT and knockout (Tlr-4 -/-) mice | DSS | - In both mice, the HU-210 promoted a reduction in the disease activity index and the histopathological score. | Lin (2017) [121] | ||
| - Decreased loss of goblet cells in the colon and ileum of WT mice. | |||||
| - In Tlr-4 -/- mice, the loss of goblet cells was less and occurred only in the colon. The HU-210 reversed the loss in this case as well. | |||||
| - Intestinal microbiota decrease in both strains of mice. | |||||
| - Decreased CD3+ T cells in the Peyer’s patches in both strains. | |||||
| - The HU-210 promoted a reduction in the ratio of CD4+/CD8+ T cells in the WT mice. A lower ratio of CD4+/CD8+ T cells was founded in Tlr-4 -/- mice. | |||||
| - Increased expression in zonula occludens-1. | |||||
| - Bacterial translocation was inhibited and plasma LPS levels were reduced. | |||||
| - Levels of inflammatory cytokines (IL-17, IL-6, IL-1β, TNF-α) were decreased in WT mice. | |||||
| - Reduced MPO activity in WT mice. | |||||
| - Positive regulation of p38a and pp38 in the colon was inhibited in the WT mice. | |||||
| WIN55,212-2 | Males CD1 mice | - | - Delayed gastric emptying. | Li (2013) [122] | |
| - Decreased intestinal motility. | |||||
| - Contractile responses generated by EFS were reduced in the ileum and colon segments. | |||||
| C57BL/6 mice (half males and half females) | DSS | - Colon weight reduced. | Feng (2016) [123] | ||
| - Plasma levels of TNF-α and IL-6 reduced. | |||||
| - Reduced MPO activity in the colon. | |||||
| - Inhibited expression of p-38 and p-p38. | |||||
| - Increased claudin-1 expression. | |||||
| CB1R agonists | |||||
| ACEA | Male CD1 mice | OM | - Colon weight gain, colon shortening, inflammation score, macroscopic damage, and diarrhea were reduced. | Kimball (2006) [102] | |
| - Almost complete resolution of epithelial damage, almost no inflammatory infiltrate, elimination of submucosal edema, and smooth muscle normalization. | |||||
| - Reduced MPO activity. | |||||
| Female BALB/c mice | DSS | - Larger doses were needed to attenuate the inflammation when compared to the inflammation induced by OM. | |||
| - Colon shortening and inflammatory damage were reduced. | |||||
| - Decreased smooth muscle thickening, normalized epithelium, and reduced inflammatory infiltrate. | |||||
| Male CD1 mice | OM | - Decreased intestinal hypermotility. | Kimball (2010) [124] | ||
| - Reduced inflammation. | |||||
| Male ICR mice peritoneal macrophages | LPS | - Nitrite production was reduced. | Romano (2013) [106] | ||
| - Absence of cytotoxic effects. | |||||
| CP55,940 | Male ICR mice | CO | - Reduced intestinal motility. | Izzo (2001) [117] | |
| - Reduced inflammation. | |||||
| CB2R agonists | |||||
| AM-1241 | C57BL/6N and knockout (CB2R -/-) mice | TNBS | - Macroscopic damage score (ulcers and colonic adhesions), colon shortening, and MPO activity were reduced. | Storr (2009) [85] | |
| - Mice with suppression in the expression of CB2 receptors do not react to treatment. | |||||
| β-Caryophyllene | Male CD1 mice | DSS, oxazolone | - Reduction of the disease activity score. | Bento (2011) [125] | |
| - Reduction of the macroscopic damages (ulcers, hyperemia, colon shortening) and the microscopic damages (bowel wall thickening). | |||||
| - MPO and NAG activity was reduced. | |||||
| - mRNA and levels colonic proteins expression of inflammatory mediators TNF-α, IL-1, IFN-γ, CXCL1/KC, and keratinocytes were reduced. | |||||
| - Increased levels of IL-4. | |||||
| - Less activation of signaling pathways involving NF-κB, CREB, ERK1/2, and IKK, induced by MAPK and transcription factors. | |||||
| - Inhibition of apoptosis and cell proliferation in colonic tissue, which was assessed by reducing the levels of caspase-3 and Ki-67, respectively. | |||||
| - The administration of the CB2R antagonist inhibited the beneficial effects of β-caryophyllene. | |||||
| Compound 26 | Mice | TNBS | - Mortality rate and body weight reduced. | El Bakali (2014) [126] | |
| - Decreased bowel thickening and ulceration areas. | |||||
| - IL-1β mRNA expression reduced. | |||||
| HU-308 | Male C57BL/6 mice | DSS | - Decreased weight loss, bloody diarrhea, colon shortening, and inflammatory infiltrate. | Ke (2016) [86] | |
| - NLRP3 proteins, casp-1 p20/casp-1 p45 ratio, mRNA proIL-1β, and IL-1β were reduced. | |||||
| - Colon SQSTM1 reduced. | |||||
| - The increased proportion of LC3-II, LC3-I, and beclin-1. | |||||
| JHW-133 | Male C57BL/6 mice peritoneal macrophages | LPS/DSS | - NLRP3 proteins, casp-1 p20/casp-1 p45 ratio, mRNA proIL-1β, and IL-1β were reduced. | ||
| - Proportion of LC3-II, LC3-I, beclin-1, and SQSTM1increased. | |||||
| - Increased level of p-AMPK. | |||||
| - Decreased level of p-mTOR and p-P70S6K. | |||||
| Male Sprague–Dawley rats | LPS | - Reduced Intestinal motility. | Mathison (2004) [52] | ||
| Males CD1 mice | OM | - Colon weight gain, colon shortening, inflammation score, macroscopic damage, and diarrhea were reduced. | Kimball (2006) [102] | ||
| - Demonstrated nearly complete resolution of epithelial damage, a near absence of an inflammatory infiltrate, elimination of submucosal edema, and normal smooth muscle appearance. | |||||
| - Reduced MPO activity. | |||||
| Female | DSS | - It required higher doses and more frequent dosing than in OM-induced colitis to decrease inflammation. | Kimball (2006) [102] | ||
| BALB/c mice | - Macroscopic damage was reduced. | ||||
| - Smooth muscle thickening decreased. | |||||
| - Epithelium normalized. | |||||
| - Inflammatory infiltrate reduced. | |||||
| C57BL/6N mice | TNBS | - Macroscopic damage score (ulcers and colonic adhesions) and colon shortening were reduced. | Storr (2009) [85] | ||
| - Reduced MPO activity. | |||||
| - Mice with CB2R suppression do not react to treatment. | |||||
| Female IL-10 -/- mice | Spontaneous chronic colitis | - Bodyweight loss was reduced. | Singh (2012) [127] | ||
| - Decreased in leukocyte infiltrates, mainly lymphocytes, in the colon's LP. | |||||
| - Partially restored glandular and goblet cell architecture. | |||||
| - Increased the expression of CD80, and CD86 by B220 cells. | |||||
| - Down-regulated the expression of 4-1BB, a T cell activation marker. | |||||
| - Decreased CD4+ T cells in the MLN and LP. | |||||
| - Increased CD4+ T cells in the spleen. | |||||
| - No change in CD8+ T cells, except in the MLN. | |||||
| - Declined CD4+CD69+T cells in the spleen, MLN and LP. | |||||
| - Reduced NK 1.1 cells in the LP. | |||||
| - Decreased mast cells in the spleen and LP. | |||||
| - Induced apoptosis of activated T cells through the action of JHW-133 in CB2R. | |||||
| - Reduced LY6G+expression in the MLN, LP, and spleen. | |||||
| C57BL/6 WT mice | DSS | - Bodyweight loss was reduced. | |||
| - Decreased lymphocyte infiltration. | |||||
| - Inflammation scores and histological damage were reduced. | |||||
| - Decreased CD11b+ expression in the MLN and LP. | |||||
| - Reduced IFN-γ in the spleen and LP. | |||||
| - Protective effect against colitis through activation CB2 receptors. | |||||
| Male ICR mice peritoneal macrophages | LPS | - Reduced nitrite levels. | Romano (2013) [106] | ||
CB1R, cannabinoid receptor 1; CB2R, cannabinoid receptor 2; WT, wild type; DSS, dextran sulfate sodium; MPO, myeloperoxidase; TNBS, 2,4,6-trinitrobenzene sulfonic acid; FAAH, fatty acid amide hydrolase; DNBS, dinitrobenzene sulphonic acid; LPS, lipopolysaccharide; IL, interleukin; TNF-α, tumor necrosis factor α; EFS, electrical field stimulation; ACEA, arachidonyl-2’-chloroethylamide; OM, oil of mustard; ICR, Institute of Cancer Research; CO, croton oil; NAG, N-acetylglucosaminidase; CREB, cAMP response element-binding; ERK1/2, extracellular signal-regulated kinase 1 and 2; IKK, Inhibitor of nuclear factor kappa-B kinase; MAPK, mitogen-activated protein kinase; NLRP2, Nod-like receptor Pyrin Domain Containing 2; SQSTM1, sequestosome 1; LC3, microtubule-associated protein light chain 3; p-AMPK, phospho-adenosine monophosphate-activated protein kinase; p-mTOR, phospho-mammalian target of rapamycin; LP, lamina propria; MLN, mesenteric lymph node.
Table 5.
| Enzyme or reuptake inhibitor | Animal | Inflammation induction | Results after administration of enzyme or reuptake inhibitor | Location of the enzymes or inhibitors | First author (year) | |
|---|---|---|---|---|---|---|
| AEA enzyme hydrolysis inhibitor | ||||||
| AA-5-HT | Male C57/BJ mice | DNBS | - Macroscopic and histological scores were reduced. | Mice colon mucosa and submucosa by liquid chromatography-mass spectrometry. | D'Argenio (2006) [97] | |
| - Reduced MPO activity. | ||||||
| - No changes in the levels of AEA and 2-AG. | ||||||
| AEA reuptake inhibitor | ||||||
| VDM-11 | Male C57/BJ mice | DBNS | - Complete reversal of colon inflammation. | Mice colon mucosa and submucosa, detected by liquid chromatography-mass spectrometry. | D'Argenio (2006) [97] | |
| - Macroscopic and histological scores were reduced. | ||||||
| - Reduced MPO activity. | ||||||
| - Increased AEA levels in the colon. | ||||||
| Male C57Bl/6N mice | TNBS | - Macroscopic and microscopic damage was reduced in the colon (ulcers and adhesions). | Mice colon, detected by real-time PCR. | Storr (2008) [87] | ||
| - Decreased colon shortening. | ||||||
| - Reduced MPO activity. | ||||||
| - Reduced mortality rate. | ||||||
| FAAH inhibitor | ||||||
| URB-597 | Male C57Bl/6N mice | TNBS | - Macroscopic and microscopic damage was reduced in the colon (ulcers and adhesions). | Mice colon, detected by real-time PCR. | Storr (2008) [87] | |
| - Decreased colon shortening. | ||||||
| - Reduced MPO activity. | ||||||
| - Reduced mortality rate. | ||||||
| Compound 39 | C57Bl/6 mice | TNBS | - Bodyweight loss was reduced. | In vitro assay with human recombinant FAAH to determine IC50. | Andrzejak (2011) [132] | |
| - Macroscopic scores were reduced. | ||||||
| - Histological scores were decreased. | ||||||
| - TNF-α and IL1-β were decreased in the colon. | ||||||
| PF-3845 | Male C57Bl/6 mice | TNBS | - Macroscopic damage score was reduced. | LC/MS/MS spectromety in mouse colon. | Sałaga (2014) [92] | |
| - Microscopic damage, such as loss of mucosal architecture, thickening of smooth muscle, abscesses in the crypts, and extensive cellular infiltration were decreased. | ||||||
| Male albino mice, FAAH -/-, C57Bl/6 background | OM | - Smooth muscle contractility was reduced in the colon. | LC/MS/MS in samples of the brainstem, ileum, and colon. | Fichna (2014) [133] | ||
| - Antidiarrheal action mediated through the CB1R. | ||||||
| - Anti-nociceptive action. | ||||||
| - No changes in the locomotor activity. | ||||||
| FAAH, COX-1, and COX-2 inhibitor | ||||||
| ARN-2508 | Male adult CD1 mice | TNBS/DSS | - Reduced inflammation. | PGF2a levels were measured in rat brain homogenates using a competitive enzyme immunoassay. | Sasso (2015) [93] | |
| - Decreased mortality rate. | ||||||
| - Bodyweight loss was decreased. | ||||||
| MAGL selective inhibitor | ||||||
| JZL-184 | Male C57Bl/6 mice | TNBS | - Macroscopic lesions are prevented in the colon. | In vitro assay in radiolabeled 2-oleoylglycerol was used as substrate for MAGL activity in mice colon samples. | Alhouayek (2011) [95] | |
| - Edema reduced in the submucosa. | ||||||
| - Mucosa structure normalized. | ||||||
| - Leukocyte infiltration reduced. | ||||||
| - mRNA levels of IL-12, IL-6, IL-1, TNF-α, IP-10 were reduced. | ||||||
| - mRNA levels of MCP-1 and MIP-1 were decreased. | ||||||
| - Plasma LPS levels were reduced. | ||||||
AEA, anandamide; AA-5-HA, N-arachidonoyl-serotonin; DNBS, dinitrobenzene sulphonic acid; MPO, myeloperoxidase; 2-AG, 2-arachidonyl glycerol; TNBS, 2,4,6-trinitrobenzene sulfonic acid; PCR, polymerase chain reaction; FAAH, fatty acid amide hydrolase; TNF-α, tumor necrosis factor α; IL, interleukin; IC50, half-maximal inhibitory concentration; LC/MS/MS, high performance liquid chromatography tandem mass spectrometry; OM, oil of mustard; CB1R, cannabinoid receptor 1; COX, cyclooxygenase; DSS, dextran sulfate sodium; PGF, prostaglandin F2a; IP-10, IFN-gamma-inducible protein-10; MCP-1, monocyte chemoattractant protein-1; MIP-1, macrophage inflammatory protein-1; LPS, lipopolysaccharide; MAGL, monoacylglycerol lipase.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download