INTRODUCTION
Although warfarin is highly effective in the prevention of ischemic stroke and systemic thromboembolism, there are several challenges to its use in clinical practice. Due to the long half-life of the agent, onset and offset of the drug effect are slow, taking 48–72 hours for complete effect to occur after administration [
1]. This slow onset and offset may raise concerns about the risk of bleeding or thrombosis when patients need to hold warfarin before and after undergoing an endoscopic or surgical procedure. Furthermore, warfarin requires frequent blood monitoring to adjust the appropriate drug dose for adequate anticoagulant effect, which can differ by more than 20 times among patients, probably due to the drug’s interaction with food and individual genetic variation [
1]. These drawbacks prompted the quest for the development of alternative drugs.
Since the Food and Drug Administration’s approval in 2010, the use of the non-vitamin K antagonist oral anticoagulants (NOACs), including dabigatran, apixaban, and rivaroxaban, has been rapidly catching up to warfarin with non-inferior efficacy [
2]. This change has been brought about by the convenience of fixed-dose regimens of NOACs with no requirement for routine laboratory monitoring owing to their predictable pharmacokinetics and pharmacodynamics [
3,
4]. However, the risk of gastrointestinal bleeding (GIB) in the use of NOACs is still in question. In pivotal trials for the efficacy and safety of NOACs, dabigatran and rivaroxaban had a higher risk of GIB than warfarin [
5,
6]. Data from real-world studies showed a low or similar risk of GIB in NOACs compared with warfarin [
7-
9]. Furthermore, the risk of GIB in NOACs over warfarin after gastrointestinal endoscopic polypectomy has yet to be fully evaluated because of the low number of anticoagulant users who undergo polypectomy.
Health administrative databases which routinely collect the health data of many subjects can provide important information about the clinical outcomes of rare events from real-world settings. The National Health Insurance Service (NHIS), an obligatory single-payer health insurance system in Korea, has a data warehouse that collects required information on insurance eligibility covering the entire population of over 50 million [
10]. Thus, using NHIS data, we aimed to determine the risk of overall and post-polypectomy GIB associated with the use of NOACs and warfarin.
DISCUSSION
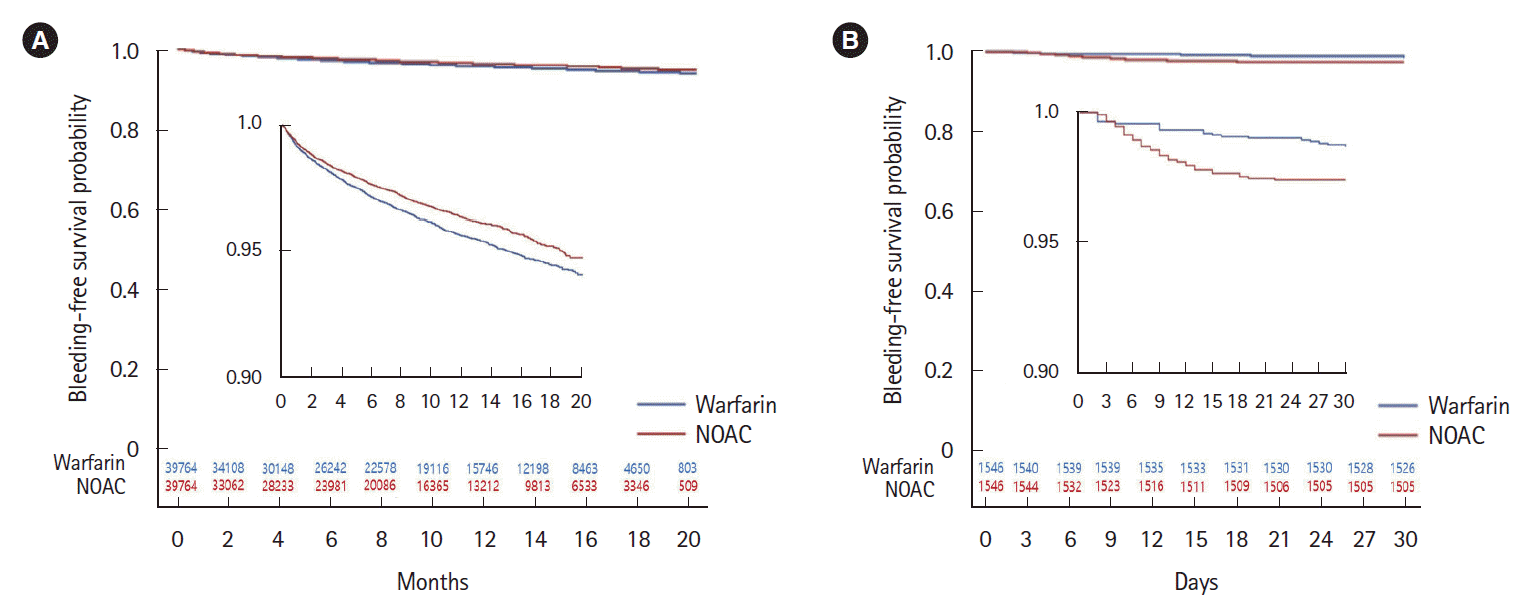
This nationwide, population-based study comparing the hazard risk for GIB between NOACs and warfarin, using propensity matching analysis, showed that overall GIB risk was lower in NOACs than warfarin, whereas post-polypectomy GIB risk was higher in NOACs than warfarin. This risk varied in different NOACs subtypes: apixaban had a lower risk of all GIB than dabigatran or rivaroxaban in the overall cohort while rivaroxaban had a higher post-polypectomy GIB risk than apixaban or dabigatran. Concomitant medications like aspirin or antiplatelets increased this risk in both the overall and post-polypectomy cohort while PPIs co-therapy with NOACs or warfarin decreased upper GIB risk, but not lower GIB risk in the overall cohort.
In the literature comparing GIB risk between NOACs and warfarin, there are conflicting results, which seem to be related to the design of the studies. Several landmark clinical trials evaluating anticoagulant efficacy for drugs have reported a higher GIB risk in NOACs than in warfarin (although GIB was not the primary endpoint of the studies) [
5,
6], while retrospective observational studies have shown a lower GIB risk or less severe GIB in NOACs than in warfarin [
7,
9,
14]. Despite uncertainty, this disparity among studies might be associated with how the therapeutic range of warfarin was controlled, which poses a great challenge in clinical practice. Compared to patients in real-world clinical settings, patients treated with warfarin in the randomized trials were much more likely to be strictly monitored, possibly leading to a lower risk of GIB [
15]. In contrast, patients in retrospective observational studies had high concentrations of warfarin, which is associated with poorly controlled monitoring; the mean international normalized ratio (INR) was over 3 and more than half of the patients showed a supratherapeutic range of INR resulting in increased risk for GIB [
15]. Our finding of excessive GIB risk in the warfarin group over the NOACs group in the general cohort is in line with those retrospective studies supporting the hypothesis that unoptimized warfarin concentration in real clinical practice (increased INR) may have potentiated bleeding from gastrointestinal mucosa in the warfarin group.
However, in the subgroup analysis of bleeding within 30 days after endoscopic polypectomy, we observed a high hazard risk in NOACs compared with warfarin. A Japanese cohort study based on a national inpatient database reported a higher risk of post-endoscopic bleeding in warfarin than NOACs users which is the opposite of our result [
12]. We believe that this difference may be attributed to several discrete features of each study. We focused on endoscopic polypectomy regardless of admission status because this procedure is commonly encountered during endoscopy amid the drastically increased volume of endoscopy screenings for cancer or precancerous lesions [
16]. In the former study, endoscopic procedures included various kinds of high-risk procedures which were performed only at inpatient settings, with polypectomy or endoscopic mucosal resection accounting for only around 20% of participants [
12]. Although the proportion of heparin bridge was not described for each group, heparin use in periprocedural management of anticoagulants might have affected GIB risk in warfarin users because heparin bridge is not recommended under NOACs treatment. Indeed, they did not find a higher risk in warfarin without heparin than in NOACs without heparin. In addition, the former study defined GIB as overt, severe bleeding requiring endoscopic hemostasis or blood transfusion, while the present study included any GIB coded with an ICD-10 diagnosis as a primary outcome. In the subgroup analysis of bleeding requiring blood transfusions, we did not find a higher risk in NOACs than warfarin, which suggests that increased risk in NOACs may be associated with minor GIB after polypectomy.
In contrast, other retrospective observational studies showed no difference between NOACs and warfarin for GIB risk after elective endoscopy [
17,
18]. One of the studies using a health care organization database reported that the cumulative incidence of GIB was higher in NOACs users compared with warfarin (
P=0.03) [
17], which is consistent with the results of our study. The exact mechanism underlying the high hazard risk of postpolypectomy GIB in NOACs over warfarin is unknown but may be partially explained by the different onset time of action for the drugs. The rapid onset of NOACs (1–4 hours) may make patients prone to bleeding from mucosal defects when administered soon after resection procedures, while the slow onset of warfarin (at least 48 hours) may allow enough time for resected sites to heal up resulting in a relatively low risk of post-polypectomy bleeding. In that regard, there is no clear consensus in the guidelines on the right time for resuming NOACs after polypectomy [
19,
20]. However, we surmised again that this increased risk in NOACs over warfarin did not apply to clinically relevant bleeding as we could not observe a difference in GIB requiring blood transfusions (
Table 4).
A recent Hong Kong study [
21] using a population-based analysis with PSM showed that apixaban was associated with a significantly lower risk of post-colonoscopic polypectomy bleeding than warfarin which appeared to be discrepant with our results. In subgroup analysis with no heparin bridging therapy, however, the study did show that the bleeding risk of post-colonoscopic polypectomy in apixaban, dabigatran and rivaroxaban was significantly higher than in warfarin group. Therefore, without heparin effect, the post-polypectomy bleeding risk might be higher in NOACs than in warfarin. Although we could not assess heparin bridge effect due to limitation of the NHIS database, there were few cases of heparin coadministration in post-polypectomy cohort (
Table 3) resulting in exclusion of heparin effect. Furthermore, rivaroxaban was significantly associated with high post-polypectomy bleeding risk compared with warfarin in multivariate analysis which was consistent with our results.
There appears to be a notable difference in the bleeding risk among NOACs subtypes. Rivaroxaban and dabigatran showed higher risk of GIB than warfarin while apixaban showed no difference in randomized trials [
5,
6,
22]. One meta-analysis with 43 randomized controlled trials found that rivaroxaban had a significantly high GIB risk while dabigatran and apixaban did not [
23]. Retrospective observational studies that compared major bleeding risk or upper GIB risk among NOACs (apixaban, dabigatran, and rivaroxaban) also reported a higher risk in rivaroxaban than in other NOACs, with apixaban showing the lowest risk [
9,
24,
25]. Likewise, we also found the highest and lowest risk of GIB in rivaroxaban in post-polypectomy cohort and apixaban in overall cohort, respectively. These individual risks for GIB in NOACs subtypes should be kept in mind for use in patients with differing GIB risks.
One of the notable findings in the present study was the effect of concomitant medications on GIB risk. Aspirin and antiplatelet agents, such as clopidogrel, ticagrelor, and prasugrel, intensified GIB risk in NOACs users; the advantage of a lower risk for GIB in NOACs over warfarin disappeared when aspirin or antiplatelets were concomitantly administered. Additionally, the hazard risk of post-polypectomy in NOACs increased when combined with these medications, especially in lower polypectomy. Considering the added risk of NOACs in conjunction with aspirin/antiplatelets, either one may be temporarily held during high-risk lower polypectomies while balancing for the risk for thrombosis.
We found that PPIs coadministration with NOACs reduced the hazard risk of overall GIB (HR, 0.70; 95% CI, 0.63–0.78) compared to NOACs alone (HR, 0.86; 95% CI, 0.77–0.97) with warfarin alone as a reference in the general cohort. In subgroup analysis, this was true for GIB requiring blood transfusions and upper GIB. This finding supported the result of a previous study of the US Medicare beneficiary database showing a significantly reduced upper GIB risk in NOACs with PPIs co-therapy [
25]. However, we did not observe the protective effect of PPIs for lower GIB, which is in line with previous knowledge demonstrating no beneficial effect of PPIs for preventing lower GIB [
26,
27]. The protective effect of PPIs was not observed in GIB after polypectomy.
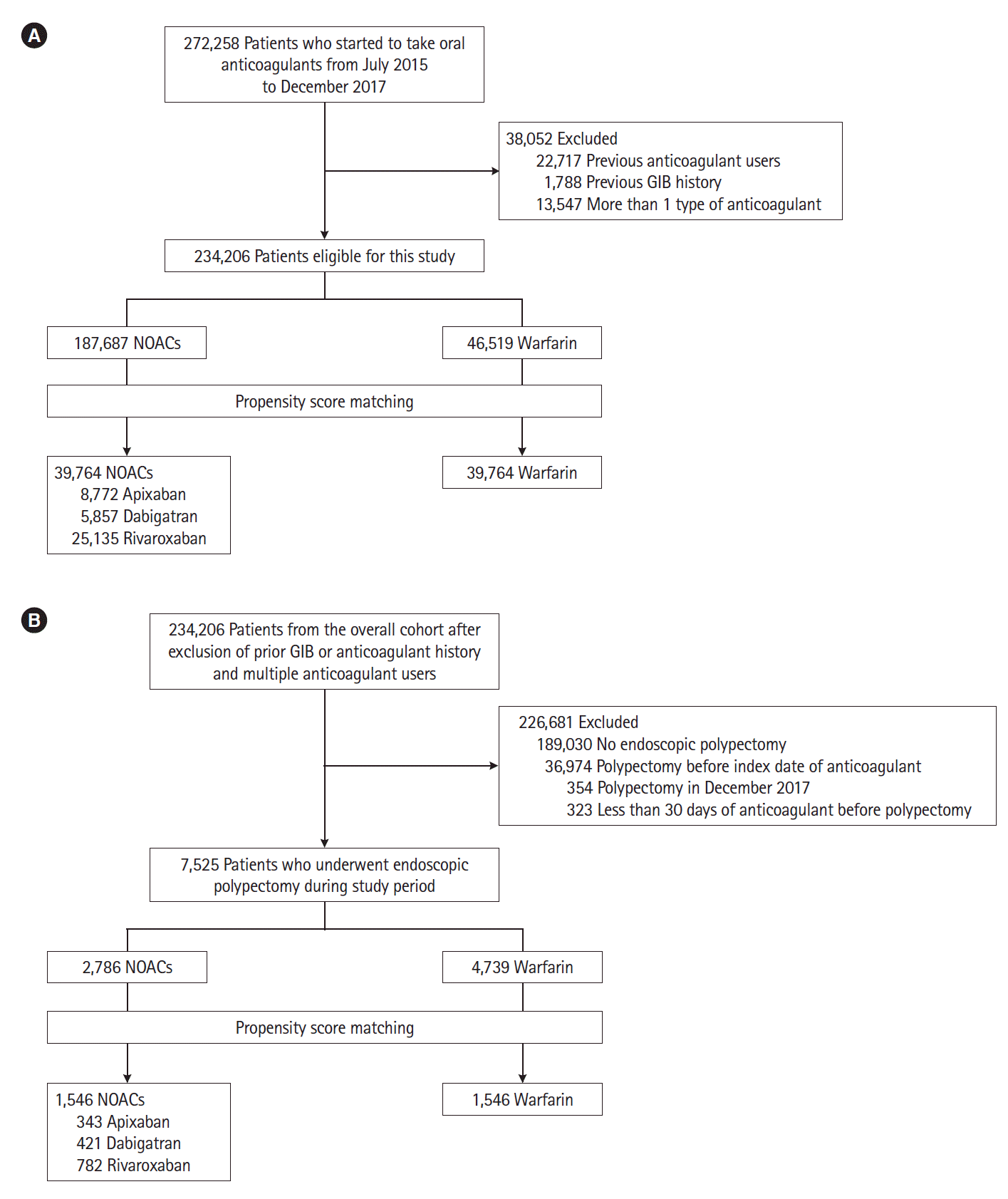
The strength of the present study is the large number of cases from a nationwide, integrated healthcare system, which enabled sufficient statistical power in discriminating the differences in GIB risk among anticoagulant users. The NHIS includes the entire population of South Korea (more than 50 million) with reimbursement claims data from primary care to tertiary hospitals, reflecting real-world clinical practice [
10]. For reducing confounders, we excluded anticoagulant exposures and patients with a previous history of GIB before the index medication start date. For the polypectomy group, we included patients who first underwent polypectomy after index medication. The limitations of the study should be noted. First, these selected patients might not be representative of the population intended to be analyzed because of a retrospective design. Second, PSM might not have controlled hidden confounding factors which could impact our results. Third, as we included GIB events and subgroups based on their diagnostic codes, procedures and filled prescriptions, there may have been misclassified or missed cases. Fourth, we could not differentiate between procedure-related and nonprocedure related GIB because we defined any GIB within 30 days after endoscopic polypectomy. Therefore, the post-polypectomy bleeding risk might have been overestimated. Fifth, the database did not provide information on the clinical data such as detailed causes of bleeding or the exact timing of drug cessation or resumption of anticoagulation especially around endoscopic procedures. Sixth, we failed to balance baseline index diseases such as atrial fibrillation and other indications between groups. Therefore, we tried to control this confounding effect on the GIB risk by adjusting index of diseases in Cox proportional hazard model. The differences in the GIB risk between groups remained significant after adjustment of this confounding factor in overall (
Supplementary Table 4) and post-polypectomy cohort (
Supplementary Table 5). Seventh, there might be a type I error risk as multiple comparison was used. Eighth, because there was no available data on thromboembolic events, when to stop before endoscopy or when to resume after endoscopy in each group, the findings did not allow us to devise optimal periprocedural management strategies for preventing post-polypectomy GIB. Finally, there was a high proportion of subjects who took NSAID in the study (over 70%) (
Table 1). If patients had the prescription of NSAID at least once during the study period, they were regarded as taking the medicine. Also, most patients were elderly and had chronic diseases which might explain the high percentage of NSAID history in the subjects.
In conclusion, this population-based comparison study using PSM demonstrates a lower risk of overall GIB but a higher risk of post-polypectomy GIB in NOACs compared with warfarin. However, the risk of post-polypectomy GIB requiring blood transfusion is not different between the groups, suggesting that an increased risk of post-polypectomy GIB in NOACs may apply only to minor bleeding. GIB risk was not same among NOACs subtypes. Concomitant aspirin or antiplatelet administration increases overall GIB and post-polypectomy GIB. PPIs co-therapy with NOACs reduces the risk of GIB in the overall cohort; this benefit of PPIs is observed in the upper gastrointestinal tract and GIB with transfusion but not lower GIB. These findings may help in choosing the optimal NOACs according to patients’ GIB risk and may suggest a need for careful monitoring for GIB after polypectomy in NOACs.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download