1. Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004; 126:451–459.

2. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018; 390:2779–2789.

3. Nielsen OH, Vainer B, Madsen SM, Seidelin JB, Heegaard NH. Established and emerging biological activity markers of inflammatory bowel disease. Am J Gastroenterol. 2000; 95:359–367.

4. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006; 55:426–431.

5. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18:2218–2224.

6. Lin WC, Wong JM, Tung CC, et al. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J Gastroenterol. 2015; 21:13566–13573.

7. Wei SC. Could fecal calprotectin enter mainstream use for diagnosing and monitoring inflammatory bowel disease? Intest Res. 2016; 14:293–294.

8. Jang HW, Kim HS, Park SJ, et al. Accuracy of three different fecal calprotectin tests in the diagnosis of inflammatory bowel disease. Intest Res. 2016; 14:305–313.

9. Elkjaer M, Burisch J, Voxen Hansen V, et al. A new rapid home test for faecal calprotectin in ulcerative colitis. Aliment Pharmacol Ther. 2010; 31:323–330.

10. Heida A, Knol M, Kobold AM, et al. Agreement between home-based measurement of stool calprotectin and ELISA results for monitoring inflammatory bowel disease activity. Clin Gastroenterol Hepatol. 2017; 15:1742–1749. e2.

11. Bello C, Roseth A, Guardiola J, et al. Usability of a home-based test for the measurement of fecal calprotectin in asymptomatic IBD patients. Dig Liver Dis. 2017; 49:991–996.

12. Vinding KK, Elsberg H, Thorkilgaard T, et al. Fecal calprotectin measured by patients at home using smartphones: a new clinical tool in monitoring patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016; 22:336–344.

13. Elkjaer M. E-health: web-guided therapy and disease self-management in ulcerative colitis. Impact on disease outcome, quality of life and compliance. Dan Med J. 2012; 59:B4478.
14. Kawashima K, Ishihara S, Yuki T, et al. Fecal calprotectin more accurately predicts endoscopic remission of Crohn’s disease than serological biomarkers evaluated using balloon-assisted enteroscopy. Inflamm Bowel Dis. 2017; 23:2027–2034.

15. Vázquez Morón JM, Pallarés Manrique H, Machancoses FH, Ramos Lora M, Ruiz Frutos C. Accurate cut-offs for predicting endoscopic activity and mucosal healing in Crohn’s disease with fecal calprotectin. Rev Esp Enferm Dig. 2017; 109:130–136.

16. Sandborn WJ, Panés J, Zhang H, Yu D, Niezychowski W, Su C. Correlation between concentrations of fecal calprotectin and outcomes of patients with ulcerative colitis in a phase 2 trial. Gastroenterology. 2016; 150:96–102.

17. Yamaguchi S, Takeuchi Y, Arai K, et al. Fecal calprotectin is a clinically relevant biomarker of mucosal healing in patients with quiescent ulcerative colitis. J Gastroenterol Hepatol. 2016; 31:93–98.

18. Dhaliwal A, Zeino Z, Tomkins C, et al. Utility of faecal calprotectin in inflammatory bowel disease (IBD): what cut-offs should we apply? Frontline Gastroenterol. 2015; 6:14–19.

19. Bjarnason I. The use of fecal calprotectin in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2017; 13:53–56.
20. Bossuyt P, Vermeire S. Treat to target in inflammatory bowel disease. Curr Treat Options Gastroenterol. 2016; 14:61–72.

21. Bossuyt P, Pouillon L, Bonnaud G, Danese S, Peyrin-Biroulet L. E-health in inflammatory bowel diseases: more challenges than opportunities? Dig Liver Dis. 2017; 49:1320–1326.

22. Bossuyt P, Pouillon L, Peyrin-Biroulet L. Primetime for e-health in IBD? Nat Rev Gastroenterol Hepatol. 2017; 14:133–134.

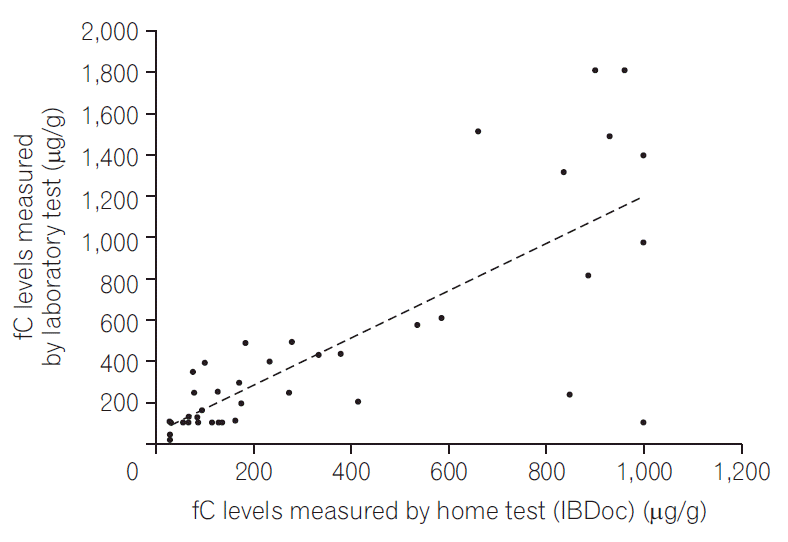
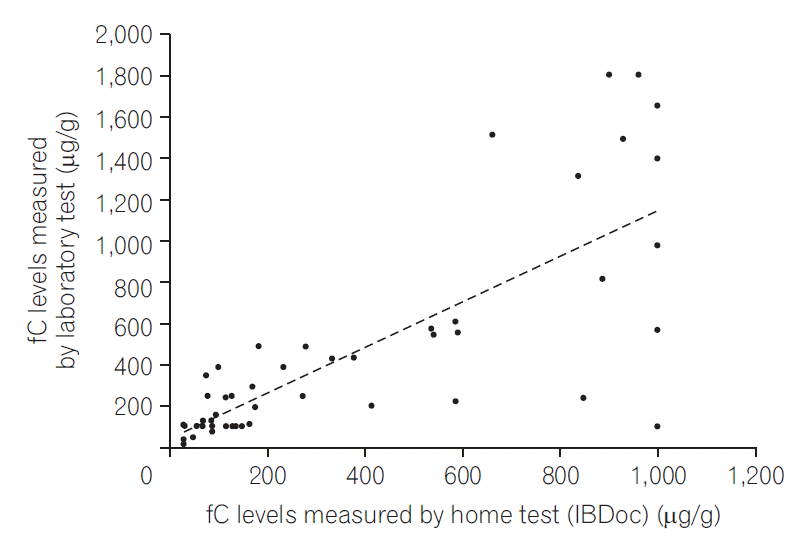
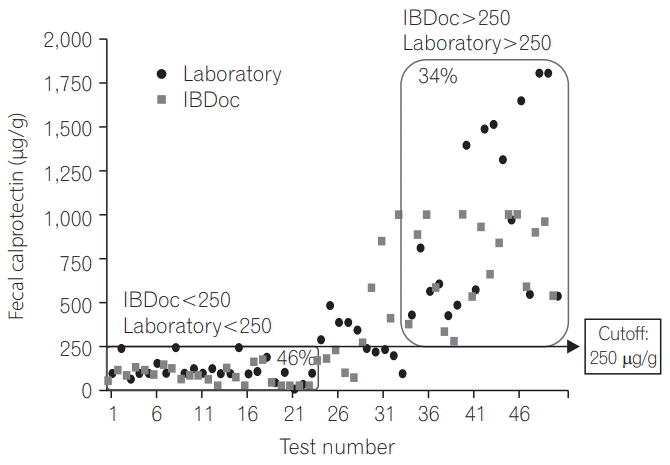
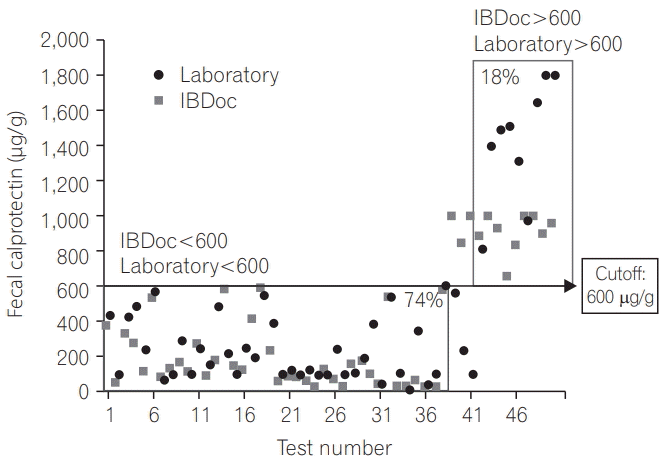




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download