Abstract
Background/Aims
Methods
Results
Notes
FINANCIAL SUPPORT
This study has not received any financial support. Although recruitment of investigators to this study was conducted in a conference called the Achievement of IBD Total Management (AIM) Jr meeting, funded by Eisai and EA Pharma, the study design, including development of the protocol, statistical analysis and drafting of the manuscript were performed only by the investigators. Therefore, this study does not have any relationship with Eisai and EA Pharma.
CONFLICT OF INTEREST
SS received lecture fees from Mitsubishi-Tanabe Pharma, Kyorin Pharmaceutical, Abbvie, EA Pharma, Mochida Pharmaceutical, Zeria Pharmaceutical, Eisai, Mylan, advisory/consultancy fees from Janssen, Kyorin Pharmaceutical, and research grant from Mitsubishi-Tanabe Pharma. TK received lecture fees from Mitsubishi-Tanabe Pharma, Eisai, Kyorin Pharmaceutical, Abbvie, Janssen, JIMRO, Ajinomoto Pharma, EA Pharma, Astellas, Mochida Pharmaceutical, Asahi Kasei Medical, Takeda Pharmaceutical, Gilead Sciences, Celltrion, Nippon Kayaku, Alfresa Pharma, Thermo Scientific, advisory/consultancy fees from Janssen, Pfizer, Kyorin Pharmaceutical, Mochida Pharmaceutical, Takeda Pharmaceutical, Eli Lilly, Ferring Pharmaceuticals, Nippon Kayaku, and research grants from EA Pharma, Thermo Scientific, and Alfresa Pharma. AY, TY, HT, and SR received lecture fees from EA Pharma. TF received research support from Eisai and lecture fees from EA Pharma. KO received research support from EA Pharma, Takeda and Daiichi Sankyo. KKak received research support from EA pharma. MN received research support from EA Pharma, Takeda, AstraZeneca and Daiichi Sankyo. TH received lecture fees from Mitsubishi-Tanabe Pharma, Kyorin Pharmaceutical, Abbvie, GK, Janssen, Mochida Pharmaceutical, JIMRO, Takeda Pharmaceutical, Gilead Sciences, Miyarisan Pharmaceutical, Kissei Pharmaceutical, Zeria Pharmaceutical, Ferring Pharmaceutical, Nippon Kayaku, advisory/consultancy fees from Mitsubishi-Tanabe Pharma, Takeda Pharmaceutical, EA Pharma, Janssen, Eli Lilly, and research grants from Abbvie, JIMRO, and Zeria Pharmaceutical. Other authors have no financial relationship to disclose. The manuscript was edited by SciTechEdit International, LLC (7012 East Mountain Brush Circle, Highlands Ranch, CO 80130, USA) without funding source.
AUTHOR CONTRIBUTION
The authors’ contributions to the study were as follows: SS, TF, SB, TK, HT, AY, YT, NK designed the study. The data were collected by SS, TF, SB, MOg, TK, MOs, HT, KO, ST, HK, KKan, SN, TS, YS, TI, KKak, AY, HY, YY, TY, AM, MN, TT, RS. SS analyzed the data and SS, TF, SB, TK, HT, AY, YT, NK interpreted the data. SS drafted the manuscript. TH made critical revisions to the manuscript. All authors reviewed and approved the final version of the manuscript.
ACKNOWLEDGMENTS
SUPPLEMENTARY MATERIAL
REFERENCES
Fig. 1.
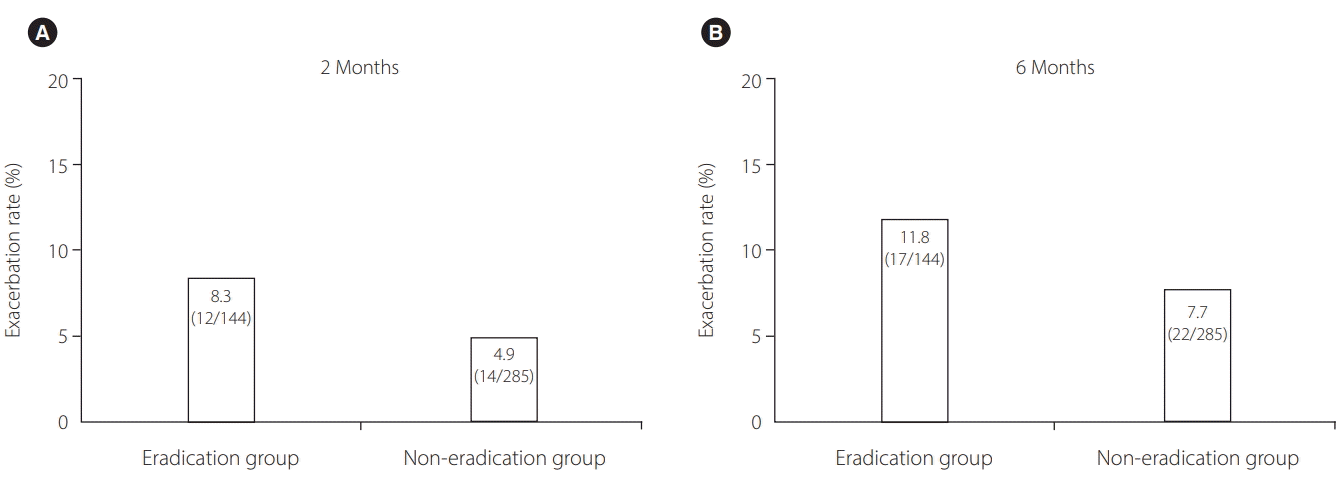
Fig. 2.
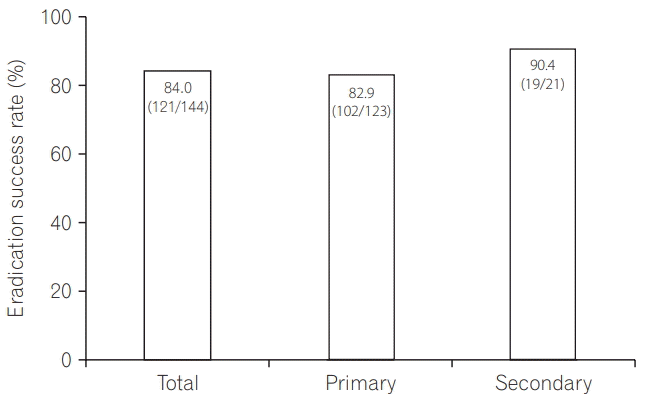
Table 1.
| Characteristic | Total (n=429) | Eradication (n=144) | Non-eradication (n=285) | P-value |
|---|---|---|---|---|
| Sex (female/male)a | 186/243 | 64/80 | 122/163 | NS |
| Age (yr)a | 51.5±13.8 | 52.6±13.9 | 51.0±13.8 | NS |
| Disease (CD/UC)a | 51/378 | 17/127 | 34/251 | NS |
| Location (CD, ileum/colon/ileocolon)a | 9-12-30 | 3-4-10 | 6-8-20 | NS |
| Location (UC, rectum/left/total)a | 126/77/175 | 42/27/58 | 84/50/117 | NS |
| Disease activity (active/remission)a | 49/380 | 17/127 | 32/253 | NS |
| Duration of disease (yr) | 10.6±9.1 | 10.5±9.4 | 10.7±9.0 | NS |
| BMI (kg/m2) | 22.5±3.6 | 22.6±3.5 | 22.5±3.6 | NS |
| Smoking (yes/no/unknown) | 57/291/81 | 17/90/37 | 40/201/44 | NS |
| Alcohol (yes/no/unknown) | 111/177/133 | 41/50/53 | 70/127/88 | NS |
| Behavior (CD, inflammatory/structuring/penetrating) | 24/17/10 | 8/6/3 | 16-11-7 | NS |
| Behavior (UC, initial/recurrent/chronic/unknown) | 39/294/37/8 | 18/94/10/5 | 21/200/27/3 | NS |
| 5-Aminosalicylic acid (yes/no) | 384/45 | 121/23 | 263/22 | 0.012 |
| Corticosteroids (yes/no) | 32/397 | 12/132 | 20/265 | NS |
| Immunomodulators (yes/no) | 90/339 | 23/121 | 67/218 | NS |
| Biologic agents (yes/no/unknown) | 43/385/1 | 9/135/0 | 34/250/1 | NS |
| Tacrolimus (yes/no) | 6/423 | 0/144 | 6/279 | NS |
| CDAI (CD) | 96±50 | 97±42 | 96±54 | NS |
| CAI (UC) | 1.2±1.1 | 1.1±1.1 | 1.2±1.1 | NS |
| Interval days between the matched cases | NA | NA | 131±450 | |
| H. pylori infection (non-eradication group, yes/eradicated/no/ unknown) | NA | NA | 7/2/89/187 |
Table 2.
| Exacerbated | Unchanged | Improved | P-value | |
|---|---|---|---|---|
| 2 Months | 0.019 | |||
| Eradication | 14 | 130 | 0 | |
| Non-eradication | 14 | 262 | 9 | |
| 6 Months | 0.362 | |||
| Eradication | 17 | 125 | 2 | |
| Non-eradication | 23 | 255 | 7 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download