Abstract
Background/Aims
In clinical practice, colonoscopy has been regarded as the gold standard for the evaluation of disease severity as well as mucosal healing in ulcerative colitis (UC). Some activity indices incorporating patient symptoms as parameters have been shown to reflect the endoscopic activity of UC. The aim of this study was to examine whether self-reported symptoms with visual analog scales (VAS) can predict endoscopic activity.
Methods
A cross-sectional study of 150 UC patients who underwent colonoscopy with submission of VAS scores of 4 symptoms: general condition, bloody stools, stool form, and abdominal pain (0: no symptoms, 10: the most severe symptoms). Each VAS score was compared with colonoscopic activity assessed with the Mayo endoscopic subscore (MES).
Results
All VAS scores were significantly correlated with the endoscopic severity (Spearman correlation coefficients of general condition, bloody stools, stool form, and abdominal pain: 0.63, 0.64, 0.58, and 0.43, respectively). Mucosal healing defined as MES 0 alone was predicted by VAS score <1.5 on general condition or 0 on bloody stools with sensitivity of 0.84 and 0.76 and specificity of 0.66 and 0.76, respectively. Additionally, VAS score <2.5 on stool form predicted active lesions in distal colorectum alone with sensitivity of 0.67 and specificity of 0.66, suggesting that this item could predict the indication of topical therapy.
Ulcerative colitis (UC) is a chronic inflammatory disease with unknown etiology that causes diffuse mucosal injuries from the rectum toward the proximal colon in continuity with a wide spectrum of severity and distribution [1]. UC patients present symptoms such as visible blood in stools, diarrhea and abdominal pain. Due to the absence of curative treatments, patients possibly live with and suffer from uncomfortable symptoms, particularly if treatment is inappropriate.
For UC management, treatment strategy should be determined based on the severity of colorectal mucosal inflammation and the extent of the disease. In addition, because mucosal healing (MH) is associated with sustained clinical remission and reduced risk of hospitalization and surgical resections, achievement of MH by UC patients has recently been pursued [2]. In general, colonoscopy has been regarded as the gold standard for the evaluation of disease activity as well as MH. Consequently, physicians are urged to frequently perform mucosal evaluations with colonoscopy. However, such an idea would be unreasonable and infeasible, because colonoscopy is burdensome for both patients and physicians. To attain surrogate colonoscopic mucosal evaluations, less invasive markers have been explored, including blood markers such as CRP [3,4], ESR [4], platelet count [5], fecal markers such as fecal calprotectin [6-9] and quantitative fecal immunochemical test (FIT) [10-12].
Meanwhile, UC involves the colorectum alone and the inflammation of the colorectum definitely causes symptoms such as diarrhea and rectal bleeding. Therefore, symptoms complained by UC patients are expected to reflect or partly parallel the activity of the disease in the colorectum. Several reports have indicated that symptoms or clinical activity indices including symptoms were correlated with endoscopic activities of UC patients [13-17]. In those reports, however, symptoms were often categorized according to the predetermined scoring index and evaluated by medical staff. If symptoms are evaluated using an index with continuous variables, more precise correlation between symptoms and endoscopic activity may be observed. Moreover, a self-reported system, if utilized sufficiently, could save labor of medical staff and medical costs.
The aim of this study was to examine whether self-reported symptoms by using visual analog scales (VAS) correlate with endoscopic activity in UC patients and their value in determining treatment strategy.
We prospectively recruited consecutive UC patients scheduled to undergo colonoscopy or sigmoidoscopy at Yokohama City University Medical Center between April and November 2015. All the patients had been diagnosed with UC using the established criteria according to endoscopic and histologic assessments. The patients were asked to indicate their symptoms using VAS on the day of colonoscopy. Patients were excluded if they were unwilling to participate in this study or considered to have bowel symptoms due to causes other than UC, including poorly controlled IBS treated with multiple anti-anxiety drugs under a psychiatrist, infectious enteritis and allergic reaction to aminosalicylate.
Information on patient demographics, disease duration, the purpose of colonoscopy, medications, and clinical disease activity was collected. Clinical remission stage was defined as a Mayo stool frequency subscore of 0 or 1 and a Mayo bloody stools subscore of 0, while the remaining were regarded as in a clinically active stage [18].
The study protocol complied with the ethical guidelines of the 1975 Declaration of Helsinki (2013 version) and was approved by the Ethics Committee of Yokohama City University Medical Center. Written informed consent was obtained from all patients.
VAS is presented as a 10-cm horizontal line between 2-endpoints, along which patients indicated a position by a vertical line according to their level of subjective assessment [19]. The VAS score is calculated by the distance from the 0 point to the patient’s mark. Symptoms were evaluated on the following 4 items in each patient: general condition, bloody stools, stool form, and abdominal pain. The scores of each item range between 0 and 10, and 0 was designated in case of completely asymptomatic, while 10 meant the most severe symptoms. In this sense, 0 in general condition was the best condition, while 10 was the worst condition. Similarly, 0 and 10 of bloody stools meant absence of blood and almost complete blood, respectively. On the stool form, 0 indicated normal form, while 10 was completely liquid diarrhea. Patients free from pain were expected to indicate 0 on the line of abdominal pain, while those who required analgesic or were unable to get up due to pain deserved to be at 10. VAS scores were counted by 0.5 in the analysis.
Bowel preparation was performed with the oral administration of a polyethylene glycol (with or without ascorbate)-based, or magnesium citrate-based electrolyte solution. After colonic lavage fluid was cleared, patients underwent colonoscopy. For severe cases that required emergency or hospitalization, sigmoidoscopy was performed without any laxative intake.
Mucosal status at each portion of the colorectum (cecum, ascending colon, transverse colon, descending colon, sigmoid colon and rectum) was assessed with the Mayo endoscopic subscore (MES) (0: normal or inactive disease, 1: mild disease with erythema, decreased vascular pattern, mild friability, 2: moderate disease with marked erythema, absent vascular pattern, friability, erosions, and 3: severe disease with spontaneous bleeding, ulceration) [20] and the maximum score in the colorectum was used for analysis. MH was defined as an MES 0 throughout the colorectum.
Besides the classification into conventional pancolitis, left-side colitis and proctitis, patients with any active lesions were stratified into 2 types according to the extent of active lesions at colonoscopy: distal colorectum alone type whose inflammation was restricted within the rectum and sigmoid colon; and descending colon or more proximal type with more extensive activity. The stratification was adopted in this study to take into consideration indications of topical therapy.
Endoscopic findings were scored using stored endoscopic images by 2 investigators (S.T. and R.K.) without any knowledge of the clinical findings or the results of VAS. When the 2 investigators disagreed on the endoscopic score for any portions, consensus was reached through the re-evaluation of endoscopic images and discussion.
SPSS software version 22.0 (IBM Corp., Armonk, NY, USA) was used for all analyses. The Spearman’s rank correlation test was performed to determine the correlation coefficient between the VAS scores and the MES. The Mann-Whitney U-test was used to determine the differences in median VAS scores. To obtain an optimal cutoff value of the VAS score, receiver operating characteristic (ROC) curve analysis was performed, and the area under the curve (AUC) was calculated. Based on the obtained optimal cutoff value of the VAS score on each item, sensitivity, specificity, positive predictive value and negative predictive value with 95% CIs were also calculated. All P-values were two-sided and considered statistically significant when <0.05.
One-hundred and sixty-six UC patients were recruited, and 16 patients were excluded (9 IBS, 4 infectious enteritis, and 3 allergic reaction to aminosalicylate). One-hundred and fifty UC patients (86 male and 64 female) who underwent colonoscopy and submitted VAS were analyzed. Their median age was 44 years (interquartile range, 33–55 years), and median duration of disease was 9 years (interquartile range, 3.7–14.8 years). More than half of the patients were in the clinical remission stage and more than one-third of the patients underwent colonoscopy for cancer surveillance (Table 1).
As for colonoscopic findings, MES 0, 1, 2, and 3 as the maximum activity throughout the colorectum were observed in 49 (33%), 41 (27%), 35 (23%) and 25 (17%) of 150 patients, respectively. Among the 132 patients (88%) with total colonoscopy, 83 had active lesions (MES >1); 35 patients with activity in the distal colorectum alone, and 48 patients in the descending colon or more proximal.
The median (interquartile range) VAS score on each symptom of the 150 examined patients was as follows: general condition, 1.8 (0.5–5.0); bloody stools, 0.5 (0.0–2.9); stool form, 2.0 (0.5–5.0); and abdominal pain, 0.5 (0.5–2.0).
The correlation between each VAS score and the endoscopic findings is shown in Fig. 1. The VAS score on each symptom increased in a stepwise manner with the elevation of MES and the correlations were statistically significant (Spearman rank correlation coefficient: general condition, 0.63; bloody stools, 0.64; stool form, 0.58; and abdominal pain, 0.43; P-values for all items <0.001). Of 11 patients who showed 0 in all 4 VAS scales, only 2 showed endoscopic activity.
Because the prognosis of patients may differ according to disease type at UC diagnosis and medications, the correlations with VAS of patients with proctitis versus non-proctitis, and of patients with aminosalicylate only versus other medications were examined (Table 2). Endoscopic findings of patients with non-proctitis (pancolitis or left-side colitis) at UC diagnosis showed correlation coefficient of around 0.60 with VAS scores of general condition, bloody stools, and stool form. Significant correlations were not observed in VAS scores except for general condition in patients with proctitis at UC diagnosis, probably due to the very small number of patients. In addition, each VAS score was moderately correlated with endoscopic findings regardless of strength of medications.
Next, the predictability of MH with VAS scores on symptoms was explored. The VAS scores of all the items were significantly lower in the patients with MH (MES 0 alone) than in those with endoscopic activity (MES 1-3) (Table 3). Because the difference in each VAS score between MES 0 and MES 1-3 was not very large, diagnostic performance of each VAS item for MH is shown in Table 4. General condition <1.5 and bloody stools=0 could discriminate patients with MH with the largest AUC value (0.82), resulting in sensitivity of 0.84 and 0.76 and specificity of 0.66 and 0.76 respectively. In our cohort, the sensitivity and specificity of partial Mayo score for MH was 0.90 and 0.60, respectively. These results suggest that patients asserting favorable general condition without any blood in stools are likely to be in MH, and that the predictability of the self-reported VAS for MH was equivalent to the partial Mayo score.
Accurate evaluation of the extent of disease activity is relevant for the optimal disease control of UC. In particular, patients with activity in the distal colorectum alone should be differentiated, because they have an indication of topical therapy. In this regard, the VAS of each item was compared between patients with activity within the distal colorectum alone and those with activity in the descending colon or more proximal. Only the VAS score on stool form showed significant difference between the 2 categories (1.5 vs. 3.0. P =0.03) (Table 5). A VAS score on stool form <2.5 discriminated patients with activity within the distal colorectum alone from those with more extensive activity with sensitivity of 0.67 and specificity of 0.66 (Table 6). These results suggest that patients for whom topical therapy is indicated are likely to defecate relatively solid stools.
The present study indicates that patient self-reported symptoms using VAS scores (on general condition, bloody stools, stool form, and abdominal pain) correlated with the endoscopic mucosal activity of UC, and that MH could be satisfactorily predicted with a low VAS score on general condition or bloody stools. In addition, a lower VAS score on stool form suggested that endoscopic activity was localized to the distal colorectum, providing an indication of topical therapy for the UC patients. Thus, self-reported symptoms of UC patients accurately reflected mucosal status, and VAS on symptoms can be a surrogate instrument for colonoscopy and can be helpful in the determination of treatment strategy.
Correlation between individual symptoms and endoscopic findings in UC patients have been investigated, particularly in reports regarding the establishment of new activity indices. In the 1980s, Powell-Tuck et al. [13] and Seo et al. [14] showed positive correlations between symptoms (5 symptoms in the former and 2 symptoms in the latter) and mucosal hemorrhage on sigmoidoscopy during the process of establishment of new activity indices. More recently, indices which include several kinds of patient symptoms as parameters have been shown to be correlated with endoscopic activity, such as the Simple Clinical Colitis Activity Index (SCCAI) with 6 symptoms (general well being, bloody stools, bowel frequency at day and night, urgency of defecation, extracolonic features) [21], the Lichtiger index with 6 symptoms (general well-being, bloody stools, abdominal pain, bowel frequency, nocturnal stools, fecal incontinence) [22], and the partial Mayo score with 2 symptoms (bloody stools and stool frequency) [23].
In contrast to those precedent indices, our system of patient self-reported symptoms with VAS has several advantages. First, it was very simple and can be evaluated as continuous variables, whereas almost all precedent indices used predetermined categorical variables, sometimes with weighting coefficients. Despite the simplicity, the correlations of the VAS with endoscopic activity were satisfactory and equivalent to those of the previous indices. Second, other indices were designed on the premise of being scored by medical staff. Moreover, several indices include physicians’ assessment as a constituent [20,24,25], which can afford inter-observer variability. Although evaluations with self-reported system could also yield variability between individuals, absence of intermediate evaluators would minimize biases and save time and cost for medical staff.
In this regard, other activity indices including blood markers such as CRP and ESR [14,24], do not have advantages over self-reported VAS with regard to both the predictive value of endoscopic activity and cost-effectiveness. Thus, we propose that patient self-reported symptoms with VAS is a simple and easy-to-perform option for the evaluation of endoscopic findings in real practice of UC.
Notably, our VAS reporting system could predict MH in UC patients. Original reports developing new indices did not refer to the correlations with MH, because the concept of MH was unfamiliar to clinicians and researchers on IBD in the era when the reports were published. In the present study, VAS scores on general condition and bloody stools were highly predictive of MH defined as MES 0. This result was in line with that of a recent report from Colombel et al. [26] that indicated absence of rectal bleeding would more likely indicate MH than stool frequency. Patients with better general condition without visible blood in stools would be expected to have achieved MH.
We rigorously defined MH as MES 0 alone, because recent reports have indicated better prognosis in patients with MES 0 than in patients with MES 1 [5,18,27]. In this regard, the results of several biomarkers using stools and blood have been reported. Fecal calprotectin, the most popular fecal marker for IBD, predicted MH as MES 0 alone with sensitivity of 0.71–0.77 and specificity of 0.72–1.00 [12,28,29]. FIT, which has recently been reported as a sensitive fecal marker for MH, showed higher sensitivity (0.93–0.94) for MES 0 alone [11,12]. Blood markers such as CRP or ESR were less sensitive with the sensitivities of around 0.70 [30]. The sensitivity of our results (0.84 for general condition and 0.76 for bloody stools) was at similar level to that of fecal calprotectin, and the specificity (0.66 for general condition and 0.76 for bloody stools) was comparable to that of FIT. As mentioned above, absolutely low cost (nearly zero cost) is a great advantage of VAS, while other markers require some costs. In this regard, physicians should note patient symptoms more diligently, given the high cost of the most popular marker fecal calprotectin, which costs more than US$100 per test.
There have been few reports evaluating the correlation between symptoms and the extent of active lesions. Moreover, no indices citing the symptom of stool form but Powell-Tuck index [13]. Our results shed light on the meaning of stool form in UC patients. From the viewpoint of physiology on stool formation in the colorectum, stools are initially watery in the proximal colon and gradually become solid toward the distal colon due to water absorption. Thus, it is reasonable to state that stools are more solid in UC patients with active lesions limited to the distal colorectum where stools already become solid than in those with extensive colitis. On the other hand, the other 3 symptoms will be presented, regardless of whether active lesions localize in the distal colorectum. Because a very low VAS score on stool form can predict MH, patients with VAS score of 0.5 to 2.5 have activity in the distal colorectum alone and good indications for topical therapy. Although we have reported that sigmoidoscopy did not always represent the most severe findings throughout the colorectum [31], VAS for stool form may help presume disease extent with sigmoidoscopy.
Based on the present results, we propose a follow-up of UC outpatients using VAS. When UC patients in the clinically remission stage have a low VAS score on general condition or bloody stools, MH is highly suggestive, and colonoscopy could be spared. When patients in the clinically active stage have a low VAS score on stool form, the extent of active lesions is probably limited to the distal colorectum, and topical therapy may be indicated for those subjects without colonoscopy. Given the good correlation with endoscopic activity, the longitudinal comparison in each patient would be useful.
The median VAS scores in the present study were relatively low, even in patients with active disease. In addition, a relatively high proportion of patients in the remission stage might make comparisons of VASs among those subjects difficult and inaccurate. In this regard, the performance should be compared between our VAS (10-point scale) and a 3- or 4-point scale such as a partial Mayo score or SCCAI among subjects in remission as well as those in active disease.
There are several limitations to this study. First, the activity of colonoscopy was evaluated with MES alone. Recently, potentially more accurate evaluations such as the Ulcerative Colitis Endoscopic Index of Severity have been suggested [32]. Validation using other endoscopic scores may be needed. In this regard, the comparison of VAS with other biomarkers such as fecal calprotectin may also be expected. Second, VAS could not be compared with histology, because biopsy was not routinely performed during colonoscopy. Recent reports suggested the clinical importance of histological remission in the disease course of UC [33,34] and thus, patient symptoms using VAS should be evaluated with histology in the future. Lastly, we excluded patients with IBS only when their symptoms were uncontrollable without psychiatric care. Therefore, our results, including relatively wide variations and low positive predictive values for MH of the VAS scales, could be largely affected by IBS symptoms of UC patients.
In conclusion, our study revealed that patient self-reported symptoms with VAS are useful to estimate endoscopic activity and to predict MH in UC patients. More importantly, physicians should be reminded that symptoms relatively accurately indicate disease and endoscopic activities and should value the importance of interviews with patients more than any other costly biomarkers.
Notes
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
AUTHOR CONTRIBUTION
Conceptualization: J.K., R.K. Methodology: J.K., R.K. Formal analysis: T.Y., M.K. Project administration: J.K. Investigation: S.T., M.M., T.O., M.N., H.K. Writing - original draft: S.T., J.K. Writing - review and editing: S.T., J.K., T.Y. Approval of final manuscript: all authors.
REFERENCES
1. Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012; 6:965–990.

2. Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010; 7:15–29.

3. Karoui S, Laz S, Serghini M, Bibani N, Boubaker J, Filali A. Correlation of C-reactive protein with clinical and endoscopic activity in patients with ulcerative colitis. Dig Dis Sci. 2011; 56:1801–1805.

4. Yoon JY, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Correlations of C-reactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig Dis Sci. 2014; 59:829–837.

5. Nakarai A, Kato J, Hiraoka S, et al. Prognosis of ulcerative colitis differs between patients with complete and partial mucosal healing, which can be predicted from the platelet count. World J Gastroenterol. 2014; 20:18367–18374.

6. Røseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997; 58:176–180.

7. Hanai H, Takeuchi K, Iida T, et al. Relationship between fecal calprotectin, intestinal inflammation, and peripheral blood neutrophils in patients with active ulcerative colitis. Dig Dis Sci. 2004; 49:1438–1443.

8. D’Incà R, Dal Pont E, Di Leo V, et al. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007; 22:429–437.

9. Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009; 15:1851–1858.
10. Kuriyama M, Kato J, Takemoto K, Hiraoka S, Okada H, Yamamoto K. Prediction of flare-ups of ulcerative colitis using quantitative immunochemical fecal occult blood test. World J Gastroenterol. 2010; 16:1110–1114.

11. Nakarai A, Kato J, Hiraoka S, et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol. 2013; 108:83–89.

12. Takashima S, Kato J, Hiraoka S, et al. Evaluation of mucosal healing in ulcerative colitis by fecal calprotectin vs. fecal immunochemical test. Am J Gastroenterol. 2015; 110:873–880.

13. Powell-Tuck J, Day DW, Buckell NA, Wadsworth J, Lennard-Jones JE. Correlations between defined sigmoidoscopic appearances and other measures of disease activity in ulcerative colitis. Dig Dis Sci. 1982; 27:533–537.

14. Seo M, Okada M, Maeda K, Oh K. Correlation between endoscopic severity and the clinical activity index in ulcerative colitis. Am J Gastroenterol. 1998; 93:2124–2129.

15. Ricanek P, Brackmann S, Perminow G, et al. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol. 2011; 46:1081–1091.

16. Turner D, Seow CH, Greenberg GR, Griffiths AM, Silverberg MS, Steinhart AH. A systematic prospective comparison of noninvasive disease activity indices in ulcerative colitis. Clin Gastroenterol Hepatol. 2009; 7:1081–1088.

17. Pagnini C, Menasci F, Festa S, et al. Application of clinical indexes in ulcerative colitis patients in regular follow-up visit: correlation with endoscopic ‘mucosal healing’ and implication for management. Preliminary results. Eur Rev Med Pharmacol Sci. 2015; 19:3674–3681.
18. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011; 141:1194–1201.

20. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987; 317:1625–1629.

21. Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998; 43:29–32.

22. Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994; 330:1841–1845.

23. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008; 14:1660–1666.

24. Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989; 298:82–86.

25. Sutherland LR, Martin F, Greer S, et al. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987; 92:1894–1898.

26. Colombel JF, Keir ME, Scherl A, et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut. 2017; 66:2063–2068.

27. Yokoyama K, Kobayashi K, Mukae M, Sada M, Koizumi W. Clinical study of the relation between mucosal healing and longterm outcomes in ulcerative colitis. Gastroenterol Res Pract. 2013; 2013:192794. doi: 10.1155/2013/192794.

28. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18:2218–2224.

29. Kristensen V, Klepp P, Cvancarova M, Røseth A, Skar V, Moum B. Prediction of endoscopic disease activity in ulcerative colitis by two different assays for fecal calprotectin. J Crohns Colitis. 2015; 9:164–169.

30. Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008; 103:162–169.

31. Kato J, Kuriyama M, Hiraoka S, Yamamoto K. Is sigmoidoscopy sufficient for evaluating inflammatory status of ulcerative colitis patients? J Gastroenterol Hepatol. 2011; 26:683–687.

32. Travis SP, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut. 2012; 61:535–542.

Fig. 1.
Correlation between visual analog scale (VAS) scores for each symptom and colonoscopic severities in the UC patients. The VAS score of each item was positively correlated with colonoscopic activity assessed with the Mayo endoscopic subscore (Spearman correlation coefficient of general condition, bloody stools, stool form, and abdominal pain: 0.63, 0.64, 0.58 and 0.43, respectively, P-values for all items <0.001).
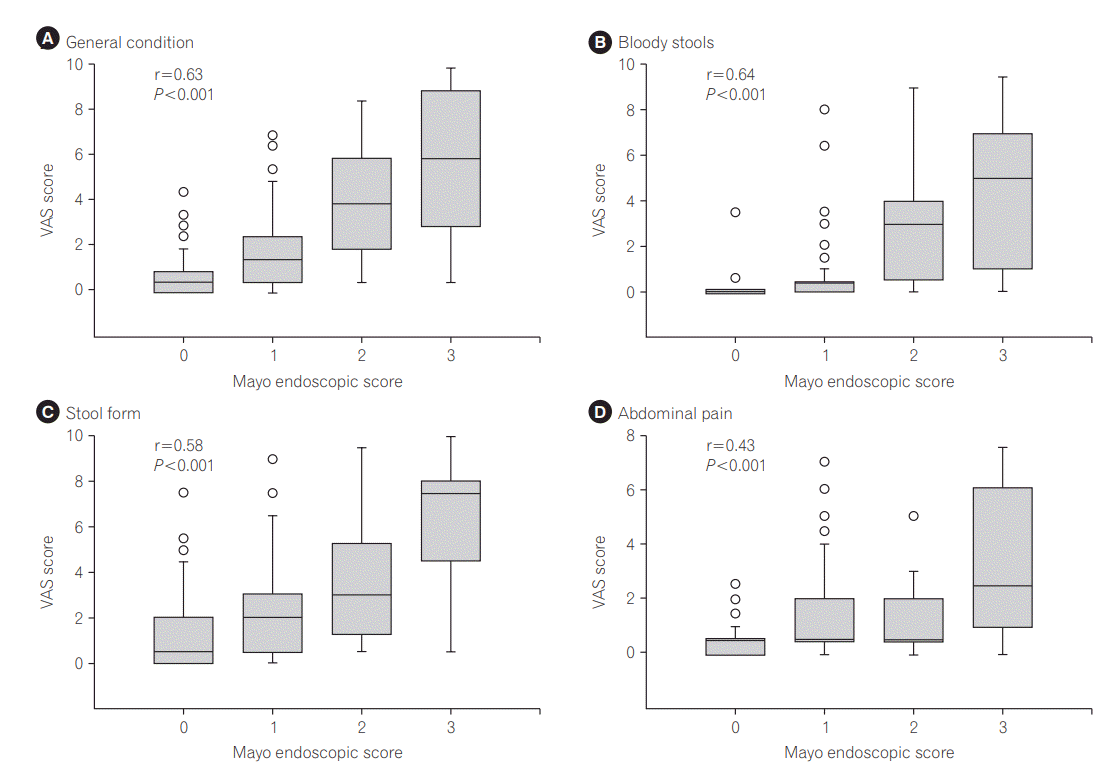
Table 1.
Characteristics of the Study Patients
| Characteristic | Value |
|---|---|
| No. of patients | 150 |
| Male sex | 86 (57) |
| Age of undergoing colonoscopy (yr) | 44 (33–55) |
| Duration of disease (yr) | 9 (3.7–14.8) |
| Clinical activity | |
| Remission stage | 84 (56) |
| Active stage | 66 (44) |
| Purpose of colonoscopy | |
| Evaluation of disease | 93 (62) |
| Surveillance | 57 (38) |
| Type of endoscopy | |
| Total colonoscopy | 132 (88) |
| Sigmoidoscopy | 18 (12) |
| Concomitant medications | |
| Aminosalicylate | 123 (82) |
| Corticosteroids | 12 (8) |
| Azathioprine/mercaptopurine | 40 (27) |
| Calcineurin inhibitor | 9 (6) |
| Anti-tumor necrosis factor α agent | 8 (5) |
| Apheresis | 2 (1) |
| Topical medications | 35 (23) |
| Others | 12 (8) |
| Analgesic use | 5 (3) |
| The maximum score of MES throughout the colorectum | |
| MES 0 | 49 (33) |
| MES 1 | 41 (27) |
| MES 2 | 35 (23) |
| MES 3 | 25 (17) |
| Disease type at UC diagnosis | |
| Proctitis | 9 (6) |
| Left-side colitis | 46 (31) |
| Pancolitis | 95 (63) |
| Extent of endoscopic activitya | |
| Distal colorectum alone | 35 |
| Descending colon or more proximal | 48 |
Table 2.
Correlations between Visual Analog Scale Scores and Mayo Endoscopic Subcore Stratified by Disease-Type or Treatment Intensity
Table 3.
Visual Analog Scale Score for Mucosal Healing versus Active Disease
Table 4.
Prediction of Mucosal Healing by Each Items of Visual Analog Scale
Table 5.
Visual Analog Scale Scores for the Extent of Active Lesions
Table 6.
Prediction of the Active Disease in the Distal Colorectum Alone by Stool Form
| Stool form <2.5a | |
|---|---|
| AUC | 0.64 |
| Sensitivity | 0.67 (0.58–0.75) |
| Specificity | 0.66 (0.53–0.77) |
| PPV | 0.73 (0.63–0.81) |
| NPV | 0.59 (0.45–0.69) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download