INTRODUCTION
Crohn's disease (CD) is a consequence of aberrant immune response to environmental factors and gut microbiome, in genetically predisposed individuals. Intestinal tuberculosis (iTB) is an infectious disease caused by
Mycobacterium tuberculosis. Despite their diverse etiologies, it is intriguing that their clinical presentation can be remarkably similar.
1 Both the entities present with chronic intestinal inflammation as ulcero-constrictive intestinal disease. As CD is being increasingly seen in TB endemic areas, differentiating between the two can be a perplexing problem for the treating clinicians.
2
3 Interestingly, granulomas in intestinal tissue are seen both in CD as well as iTB.
1 Another intriguing aspect is that mucosal epithelioid granulomas is among the histological parameters often reported to determine the clinical severity in CD. Patients with granulomas in CD hves been shown to have more clinical severity, recurrences, frequent fistula formation and postsurgical complications than in granuloma-negative CD.
4
5
Innate as well as adaptive immune responses play an important role in pathogenesis of CD as well as iTB. Over the last decade, it has been seen that the influence of innate immune response is possibly more as compared to adaptive immune response.
6
7
8
9
10 Reciprocal interactions between macrophages and activated T-cells trigger adaptive immune responses and cytokines released by both the macrophages and the activated T-cells, further modulate each other. Gradually, the concept of this reciprocal activation between macrophages and T-cells has expanded and it has been seen that the interferon γ (IFN-γ) released by type 1 T helper (Th1) cells and interleukin 4 (IL-4) released by the Th2 cells, activates 2 specific types of macrophage populations, the M1
φ and M2
φ, respectively.
11
12
13
14
15
16 Mantovani and colleagues gave a clear concept regarding the stimulators and effects of M1
φ (IFN-γ combined with lipopolysaccharide [LPS] or tumor necrosis factor [TNF]) and M2 (IL-4 [M2a]; IL-10 and GCs [M2c]).
17 On the other hand, macrophage subtype responsible for immune regulation, stimulated by Fc receptors and immune complexes, were termed as M2b
φ by Moser and Murphy.
10 Granulocyte-macrophage colony stimulating factor (GM-CSF) and macrophage colony stimulation factors (M-CSF) were identified as independent activators of M1 and M2
φ.
18
19 Recently, we showed that classical activation of macrophages using IFN-γ alone or in combination with LPS results is increased mitochondrial depolarization, lower autophagy and increased cellular reactive oxygen species leading to activation of inflammasome pathway.
20 This allowed us to envisage an intriguing hypothesis to test whether macrophages from CD patients could respond more aggressively to a common inflammatory stimulus as against those from iTB patients.
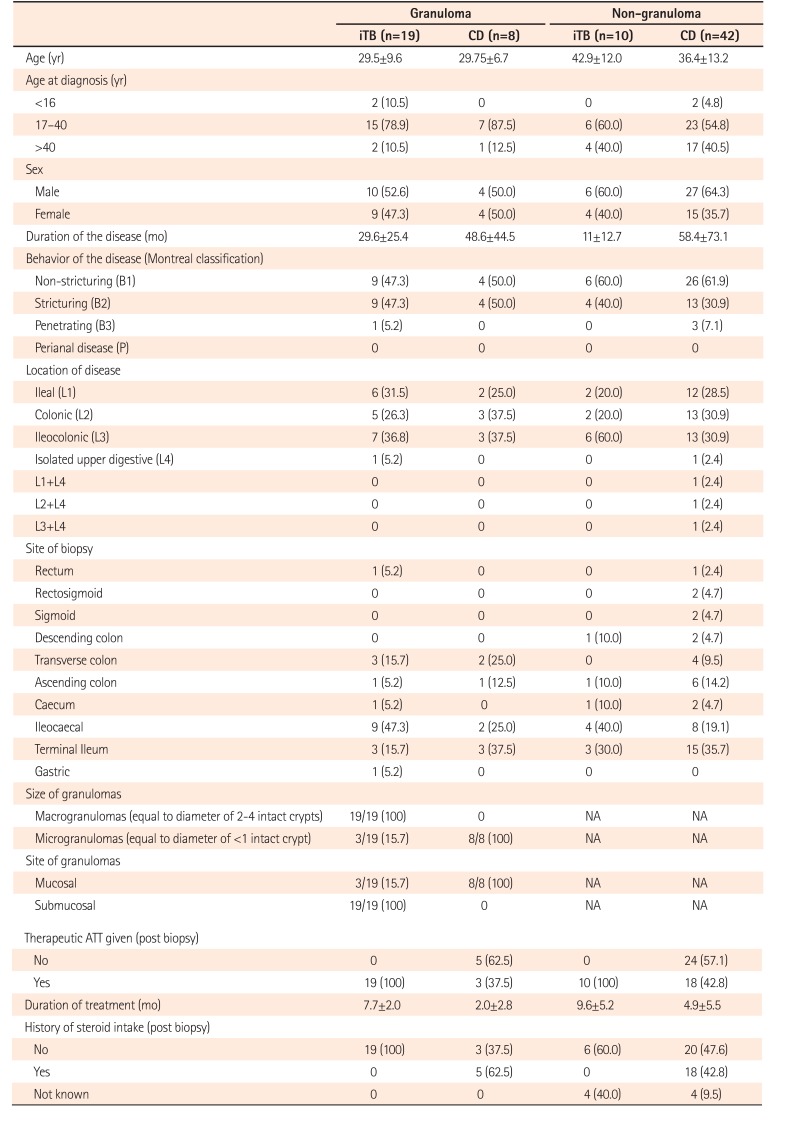
As, both CD and iTB show histological presence of mucosal lymphomononuclear cells, along with macrophages, polymorphs and epithelioid cell granulomas in a sub-set, we intended to characterize the mucosal M1φ and M2φ polarization in them. Keeping in mind, that histologically presence of epithelioid cell granuloma in CD, may be an indicator of clinical disease severity, we included cases of both granuloma-positive and granuloma-negative CD, along with iTB and control intestinal tissue, and compared the pattern of φpolarization pattern among these clinical groups.
Go to :

DISCUSSION
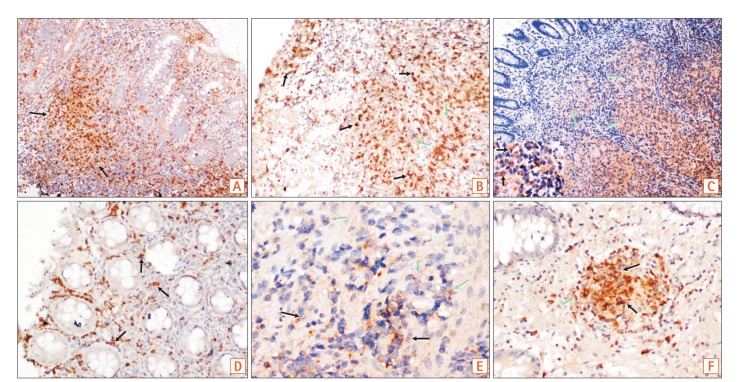
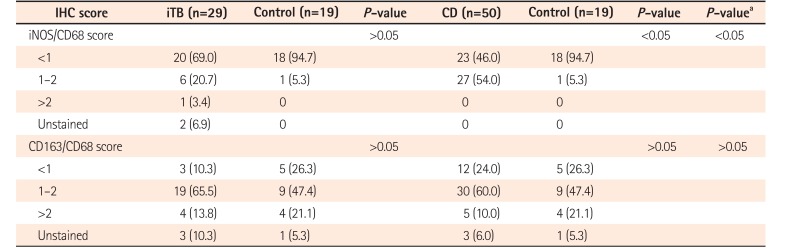
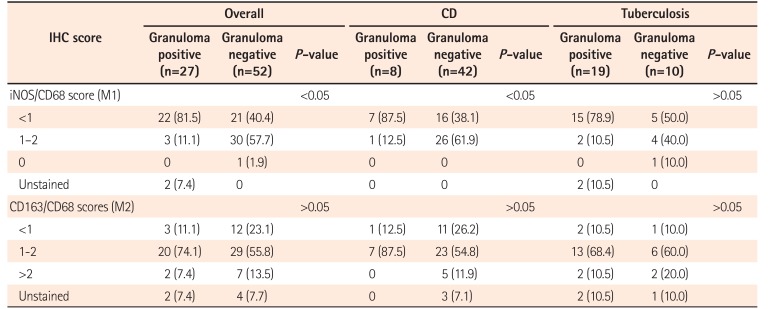
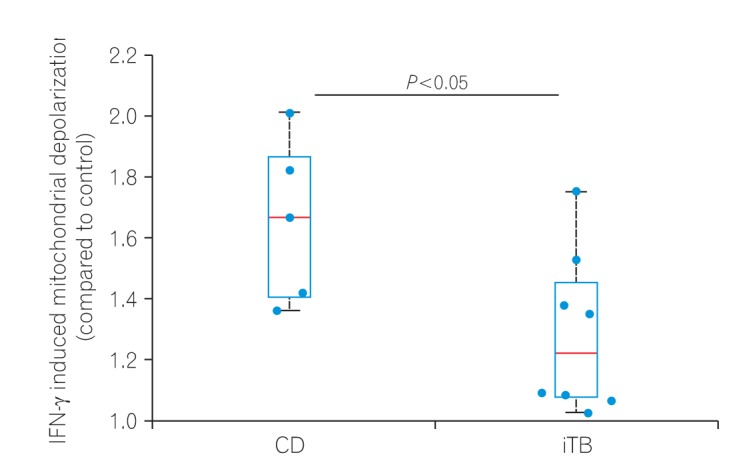
In this study, colonic biopsies from CD, iTB and control cases were subjected to IHC staining to see differential polarization pattern of M1φ and M2φ, if any. We noticed pro-inflammatory M1φ polarity both in biopsies of CD and iTB, in comparison to the controls. In comparison to iTB, in CD, the M1φ polarization was more. Furthermore, in biopsies showing histological granulomas, were also found to have more M1φ polarization, in comparison to biopsies without histological evidences of granulomas. The latter pattern was especially significant in biopsies from patients with CD than in iTB. On in vitro study, the mononuclear cell derived macrophages from both CD and iTB patients were stimulated with IFN-γ and macrophage membrane depolarization was assessed by JC-1 (mitochondrial membrane potential probe) dye, to validate our observation in human tissues. The in vitro results corroborated with the histopathology data as we observed heightened mitochondrial depolarization of macrophages from patients with CD, than in iTB, upon IFN-γ stimulation.
Though absolute specific phenotypical markers for M1 and M2 macrophages practically do not exist, various helpful markers have been identified, as CD38, Gpr18 and Fpr2 as novel M1 markers and Egr2, c-Myc as useful M2 markers. A CD38/Egr2-based flow cytometry assay is capable of distinguishing M1 and M2 macrophages efficiently,
23 however, in tissues CD38 would have also expressed on differentiating B cells. Hence, in human intestinal biopsies, we chose to use iNOS for identifying the M1 macrophages, as it is highly expressed by M1
φ. For identifying M2 macrophages we used a combination of CD163/CD68, though there are controversies that if CD163 alone can identify M2 macrophages specifically if CD163 is a marker of macrophages as a whole.
24 CD163 is a scavenger receptor and is seen on macrophages in many organs, except splenic white pulps. However, in several studies CD163 had been used as a marker of M2 macrophages.
24
25 Our primary aim was to see the polarization pattern of M1 macrophages in CD, in comparison to iTB, hence, as a proof of concept study, we used these combinations. Differentiation of a macrophage-dendritic progenitor cell is dependant on the transcription factors as PU.1 and M-CSF.
26 The M-CSF receptor is a tyrosine kinase transmembrane receptor, which on activation leads to receptor dimerization, autophosphorylation, PI3-kinase activation and eventually nuclear translocation of transcription factors. M-CSF mutant mice while showed reduced levels of circulating monocytes and selected macrophages; in human lack of M-CSF function leads to myelodysplastic syndrome or acute myeloid leukemia.
19
27 For
in vitro study, we differentiated MDMs from peripheral blood mononuclear cells, collected from both CD and iTB patients, with the help of M-CSF, and then examined the macrophage membrane potential with JC-1 stain, by stimulating them with IFN-γ. Using this method for measuring the macrophage membrane potential for liposomes is time tested and reliable.
28
In steady state, intestinal macrophages remain in M2 state, as identified by CD206 and CD163, which produce IL-10, IL-1β and transforming growth factor-β and help in proliferation of epithelial cells and proliferation of T-regulatory cells.
26 Qualls and colleagues had shown that depletion of M2 macrophages in an experimental model, causes severe colitis, indicating antagonizing effects of M2 macrophages in intestinal inflammation.
29 Tamoutounour and colleagues had demonstrated in a mouse model of T-cell mediated colitis that, the M1 type of pro-inflammatory macrophages become predominant after 12 hours of transfer and remain as the dominant cells till 3 weeks post-transfer. Whether the M2 macrophages, or the dendritic cells themselves can be converted into M1 type of cells during colitis is not known.
30 Thiesen et al.
31 had demonstrated more number of CD14 HLA-DR macrophages in inflammed colon in CD. Magnusson et al.,
32 also noted a similar finding. Kamada et al.
33 had identified CD14
+CD33
+ macrophages in CD, which secreted more IL-23 and TNF-α, on stimulation by
Escherichia coli. Almost half of the colonic macrophages in CD was found to be CD14 positive. Ogino and colleagues observed CD14+ CD163
low macrophages, which had properties similar as Th1 lymphocytes.
34 Similar observations were also made in UC. Whereas in some study a M2 prominent macrophage pattern was noted in UC, in contrast to the CD,
26 in a study by Lissner et al.,
35 predominant proinflammatory M1
φ reaction was noted in UC. In the latter study, in an
in vitro co-culture model, the authors had demonstrated that the M1
φ possibly causes apoptotic cell damage of the intestinal epithelial cells and increase trans-epithelial permeability, by altering the tight junction proteins.
35 Another interesting fact is that in CD, the tissue infiltrating macrophages may have defective function, as a result
E. coli strain have been demonstrated within macrophages in granuloma positive CD; which is usually not the fact in UC.
36 Moreover, studies on macrophage polarization in iTB is sparse, hence, our primary objective to analyze the macrophage polarization pattern between CD and iTB was novel. A literature search revealed M2 macrophage polarization in lung TB by stimulating the WNT6 signaling pathway.
37 It was noted that,
M. tuberculosis secretes virulence factors such as lipo-arabinomannan and early secretory antigenic target-6, which inhibit M1 activation.
38 However, in intestinal TB no such report is available.
As iNOS production is highly enhanced in M1 macrophages, our observations on biopsy and IHC were supported by the findings of
in vitro analysis. As mentioned previously, use of CD163 alone as a marker of M2 macrophages is debatable. Being a scavenger receptor, it may reflect generalized macrophage activation. However, soluble CD163 in blood, cleaved from macrophage surface by matrix mettalo-proteases, also had been used as a marker of macrophage activation in literature.
36 In a study by Jablonski et al.,
39 the authors specifically addressed this issue and tried to identify most specific marker for M1 and M2 type of macrophages by comparative transcriptional analysis, followed by flow cytometry analysis. They noted that CD38 labeled 71% of M1 macrophages, Erg2 labeled 70% of the M2 macrophages. However, many more markers have been described in various studies as CD38, Gpr18 and Fpr2 as novel M1 markers and Egr2, c-Myc as useful M2 markers which show relative specificity. We acknowledge therefore that a more robust panel of markers could be used to conclusively rule out any enrichment pattern of M2 macrophages. Morever, this study also needs to be expanded to a wider number of samples in future. In iTB, while M1
φ polarization, was definitely less than that in CD, overall, in granuloma-positive cases, M1
φ polarization was significant, possibly indicating more severe intestinal pro-inflammatory change. In granuloma-positive CD, the clinical disease severity, number of recurrences, fistula formation, stricture, postsurgical complications are known to be more, than in the granuloma-negative CD. Looking at our observation, it appears that, due to more pro-inflammatory activity in intestine in granuloma-positive CD patients these complications are prevalent. This is one of the novel observations of this study. Due to the same reason, CD, as a whole appears to be a more notorious inflammatory lesion of intestine, than that of iTB, especially, in terms of chronicity and response to treatment concerned. We again highlight the fact that, while the M1
φ was significantly more in granuloma-positive CD than the granuloma-negative CD, between granuloma-positive and negative iTB, similar difference was not seen. However, it is needed to be highlighted that, M2 macrophage numbers are also increased in both CD and iTB, than the controls (though not statistically significant). Classically M2 macrophages are considered necessary for development of fibrosis and wound healing. M2 macrophages, have also been shown to reduce the overall local intestinal immunity, leading to development of disease recurrence and opportunistic infections.
35
36
Hence, this study provides a preliminary insight on the fact that, M1φ are significantly polarized in intestinal mucosa of CD, then in iTB. M1φ polarization is further prominent in chronic intestinal inflammation with presence of granulomas. Since MDMs from CD showed heightened inflammatory response than those from iTB upon encountering inflammatory stimuli, the study also, albeit preliminarily, suggest inherent innate defense mechanism as the possible key player in the pathology of CD. This knowledge, not only enriches us regarding complex pathogenesis of intestinal inflammatory conditions, but also shows hope in using M1φ targeted therapeutic agents in near future. Presence of more pro-inflammatory M1φ in intestinal wall in granuloma-positive CD might explain the disease severity, recurrent, fistula, postsurgery complications already reported in this subset of CD.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download