Abstract
Background/Aims
Methods
Results
Conclusions
ACKNOWLEDGEMENTS
Notes
FINANCIAL SUPPORT: The study was supported by Mochida Pharmaceutical Co., Ltd. Mochida Pharmaceutical Co., Ltd. provided funding to support the provision of multimatrix mesalazine (Shire US Inc.) and time-dependent controlled-release mesalazine (Kyorin Pharmaceutical Co., Ltd.).
CONFLICT OF INTEREST: H.O. has received consulting, grant, or lecture fees from Mochida Pharmaceutical Co., Ltd., JIMRO, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Kyorin Pharmaceutical, Otsuka Pharmaceutical, Astellas Pharma, Eisai, Zeria Pharmaceutical, AbbVie G.K., EA Pharma, and Boston Scientific Japan K.K. S.M. and A.H. are employees of Mochida Pharmaceutical Co., Ltd. T.H. is editor-in-chief of Intestinal Research and has received consulting, grant, lecture, or manuscript preparation fees from Mochida Pharmaceutical Co., Ltd., AbbVie G.K., EA Pharma, AstraZeneca K.K., JIMRO, Mitsubishi Tanabe Pharma, Eisai, Takeda Pharmaceutical, Zeria Pharmaceutical, Janssen Pharmaceutical K.K., Astellas Pharma, and Otsuka Pharmaceutical.
Mochida Pharmaceutical Co., Ltd. provided funding to support the provision of multimatrix mesalazine (Shire US Inc.) and time-dependent controlled-release mesalazine (Kyorin Pharmaceutical Co., Ltd.).
AUTHOR CONTRIBUTION: All the authors participated in drafting of the manuscript or its critical revision. All the authors had full access to all data related to the study and assume final responsibility for the decision to submit the paper for publication. Study design, study supervision, analysis and interpretation of data, principal investigator (H.O.); patient recruitment, data collection (T.Y.); study concept and design, data analysis and interpretation (S.M.); study concept and design, statistical analysis and interpretation (A.H); study design, study supervision, analysis and interpretation of data, principal investigator (T.H).
References
SUPPLEMENTARY MATERIAL
Supplementary Information of Each Investigator
Fig. 1
Patients disposition. aMultiple selection is allowed as the reason for discontinuation. Multimatrix-2.4, multimatrix mesalazine 2.4 g/day once daily; Multimatrix-4.8, multimatrix mesalazine 4.8 g/day once daily; Time-2.25, time-dependent (controlled-release) mesalazine 2.25 g/day 3 times daily. FAS, full analysis set; UC-DAI, UC Disease Activity Index; PPS, per protocol set.
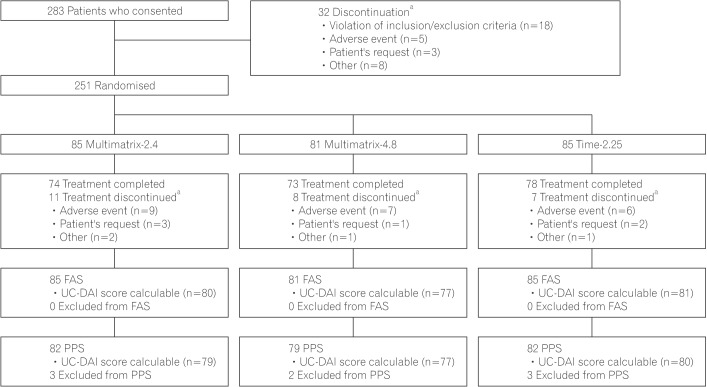
Fig. 2
Change in UC Disease Activity Index (UC-DAI) score. The mean change in UC-DAI score and two-sided 95% CI in the full analysis set (FAS) was −3.3 (−3.9 to −2.8) in the Multimatrix-4.8 group, −1.9 (−2.5 to −1.3) in the Multimatrix-2.4 group, and −2.4 (−3.0 to −1.7) in Time-2.25 group. Error bars indicate 95% CI. Multimatrix-4.8, multimatrix mesalazine 4.8 g/day once daily; Multimatrix-2.4, multimatrix mesalazine 2.4 g/day once daily; Time-2.25, time-dependent (controlled-release) mesalazine 2.25 g/day 3 times daily.
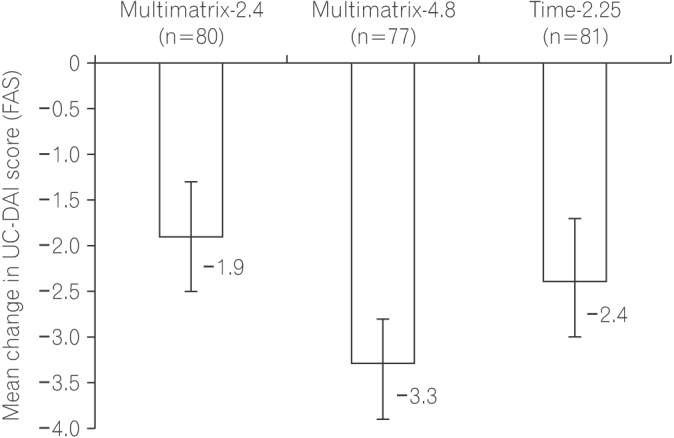
Table 1
Patient Demographics (FAS)
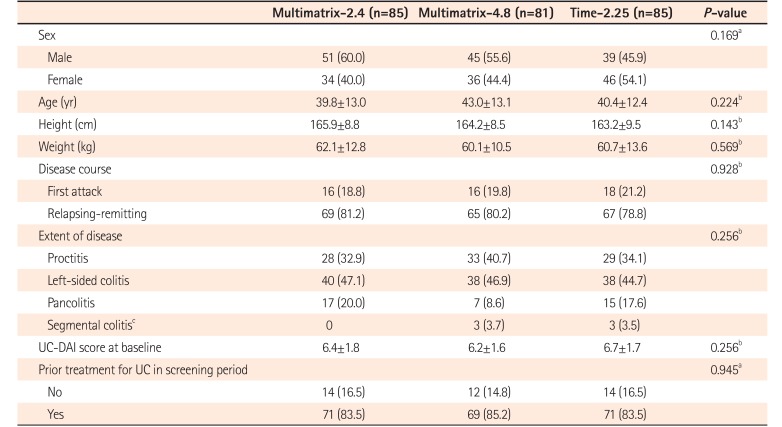
Values are presented as number (%) or mean±SD.
aChi-square test.
bANOVA.
cPatients with right-sided inflammation in the skip lesion or rectal sparing, and with mucosal findings within at least the range from the rectal to sigmoid colon for UC Disease Activity Index (UC-DAI) scoring were enrolled.
FAS, full analysis set; Multimatrix-2.4, multimatrix mesalazine 2.4 g/day once daily; Multimatrix-4.8, multimatrix mesalazine 4.8 g/day once daily; Time-2.25, time-dependent (controlled-release) mesalazine 2.25 g/day 3 times daily.
Table 2
Change in UC-DAI Score (at End of Treatment)
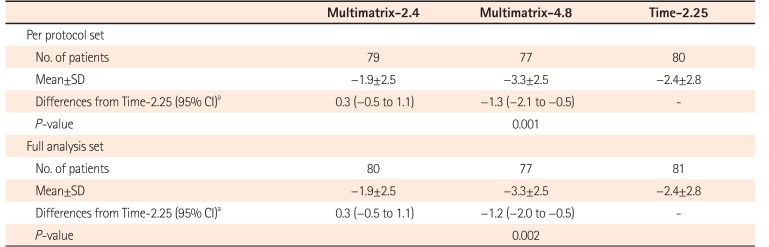
Change in UC-DAI score=UC-DAI score at the end of treatment-UC-DAI score at baseline.
aDifferences in the mean change in UC-DAI score adjusted for UC-DAI score at baseline between groups.
UC-DAI, UC Disease Activity Index; Multimatrix-2.4, multimatrix mesalazine 2.4 g/day once daily; Multimatrix-4.8, multimatrix mesalazine 4.8 g/day once daily; Time-2.25, time-dependent (controlled-release) mesalazine 2.25 g/day 3 times daily.
Table 3
Remission, Clinical Remission, Endoscopic Remission and Improvement (FAS)
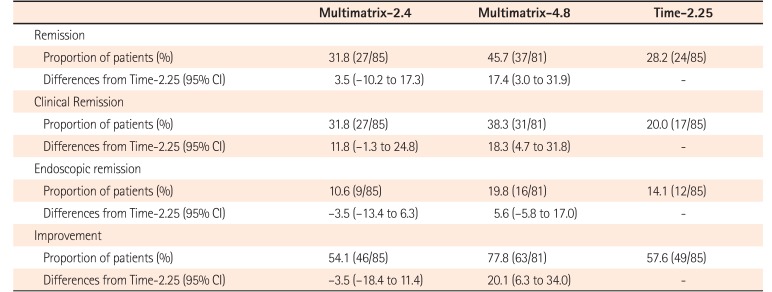
Remission: UC Disease Activity Index (UC-DAI) ≤2 and rectal bleeding score=0; clinical remission: rectal bleeding score=0, stool frequency score=0; endoscopic remission: sigmoidoscopy score=0; improvement: decrease of ≥2 points from baseline in UC-DAI.
FAS, full analysis set; Multimatrix-2.4, multimatrix mesalazine 2.4 g/day once daily; Multimatrix-4.8, multimatrix mesalazine 4.8 g/day once daily; Time-2.25, time-dependent (controlled-release) mesalazine 2.25 g/day 3 times daily.
Table 4
Change in UC-DAI Score Variables (FAS)
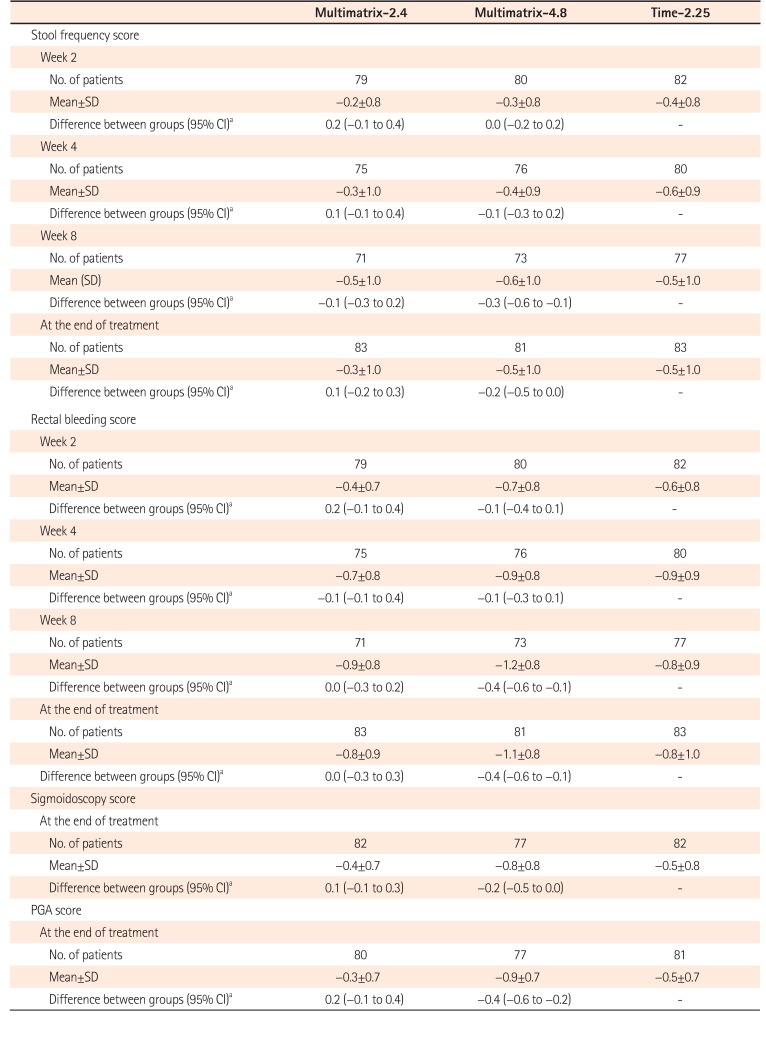
Change in each score=score at each evaluation point-score at baseline.
aDifferences in mean change in scores adjusted for scores at baseline between groups (multimatrix mesalazine-time-dependent release mesalazine).
UC-DAI, UC Disease Activity Index; FAS, full analysis set; Multimatrix-2.4, multimatrix mesalazine 2.4 g/day once daily; Multimatrix-4.8, multimatrix mesalazine 4.8 g/day once daily; Time-2.25, time-dependent (controlled-release) mesalazine 2.25 g/day 3 times daily; PGA, physician's global assessment.
Table 5
Change in UC-DAI Score by Subgroup (FAS)
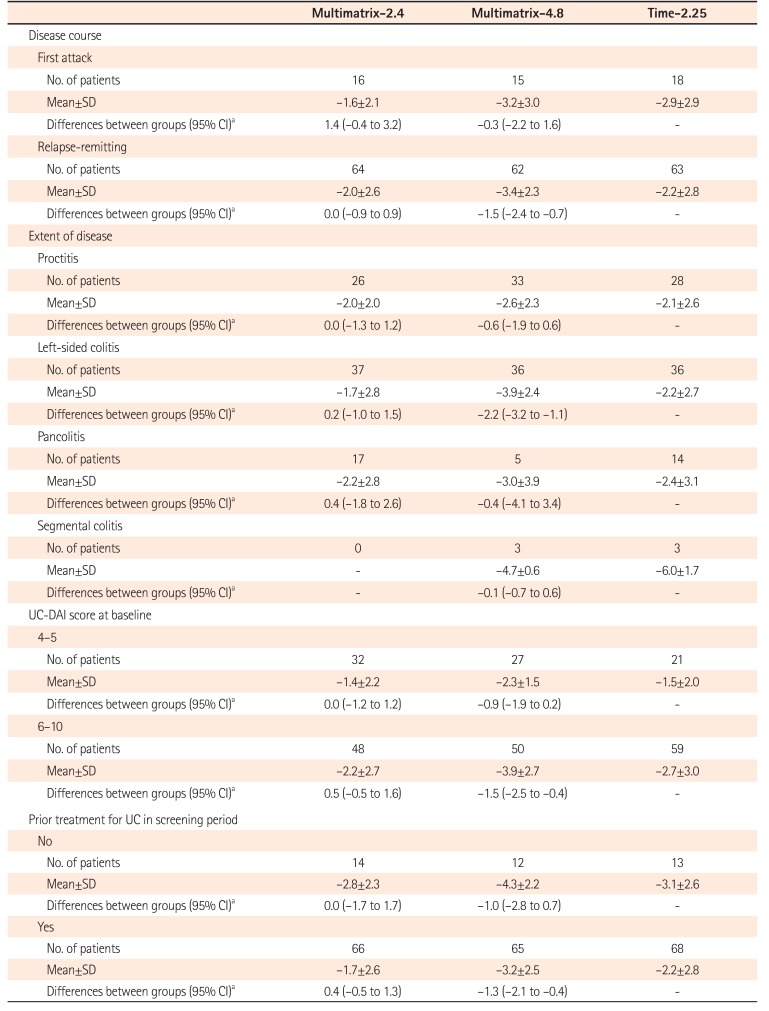
Change in UC-DAI score=UC-DAI score at the end of treatment- UC-DAI score at baseline
aDifferences in mean change in UC-DAI score adjusted for the baseline values between groups (multimatrix mesalazine-time-dependent release mesalazine).
UC-DAI, UC Disease Activity Index; FAS, full analysis set; Multimatrix-2.4, multimatrix mesalazine 2.4 g/day once daily; Multimatrix-4.8, multimatrix mesalazine 4.8 g/day once daily; Time-2.25, time-dependent (controlled-release) mesalazine 2.25 g/day 3 times daily.
Table 6
Incidence of Adverse Events
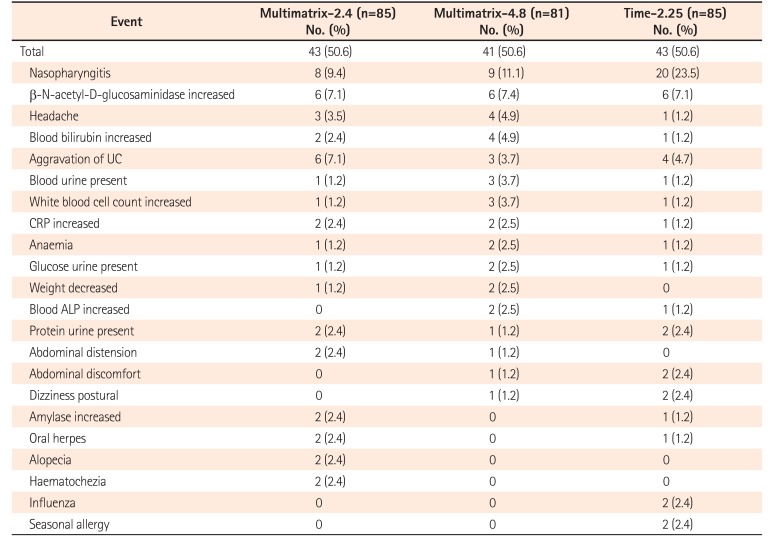
Adverse events reported by at least 2% of patients in any treatment group during the treatment period are listed.
Multimatrix-2.4, multimatrix mesalazine 2.4 g/day once daily; Multimatrix-4.8, multimatrix mesalazine 4.8 g/day once daily; Time-2.25, time-dependent (controlled-release) mesalazine 2.25 g/day 3 times daily.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download