Abstract
Methods
Patients with moderate-to-severe active steroid-refractory ulcerative colitis (UC) treated at Dayanand Medical College and Hospital, India were offered cyclosporine A, biologicals or biosimilars, or surgery. A retrospective analysis was conducted on patients who were treated with the adalimumab biosimilar, Exemptia. These patients were administered an induction dosing schedule of 160 mg Exemptia at week 0, 80 mg at week 2, and then 40 mg every other week from week 4 to 8. The clinical response and remission were assessed at week 8 using Mayo score.
Results
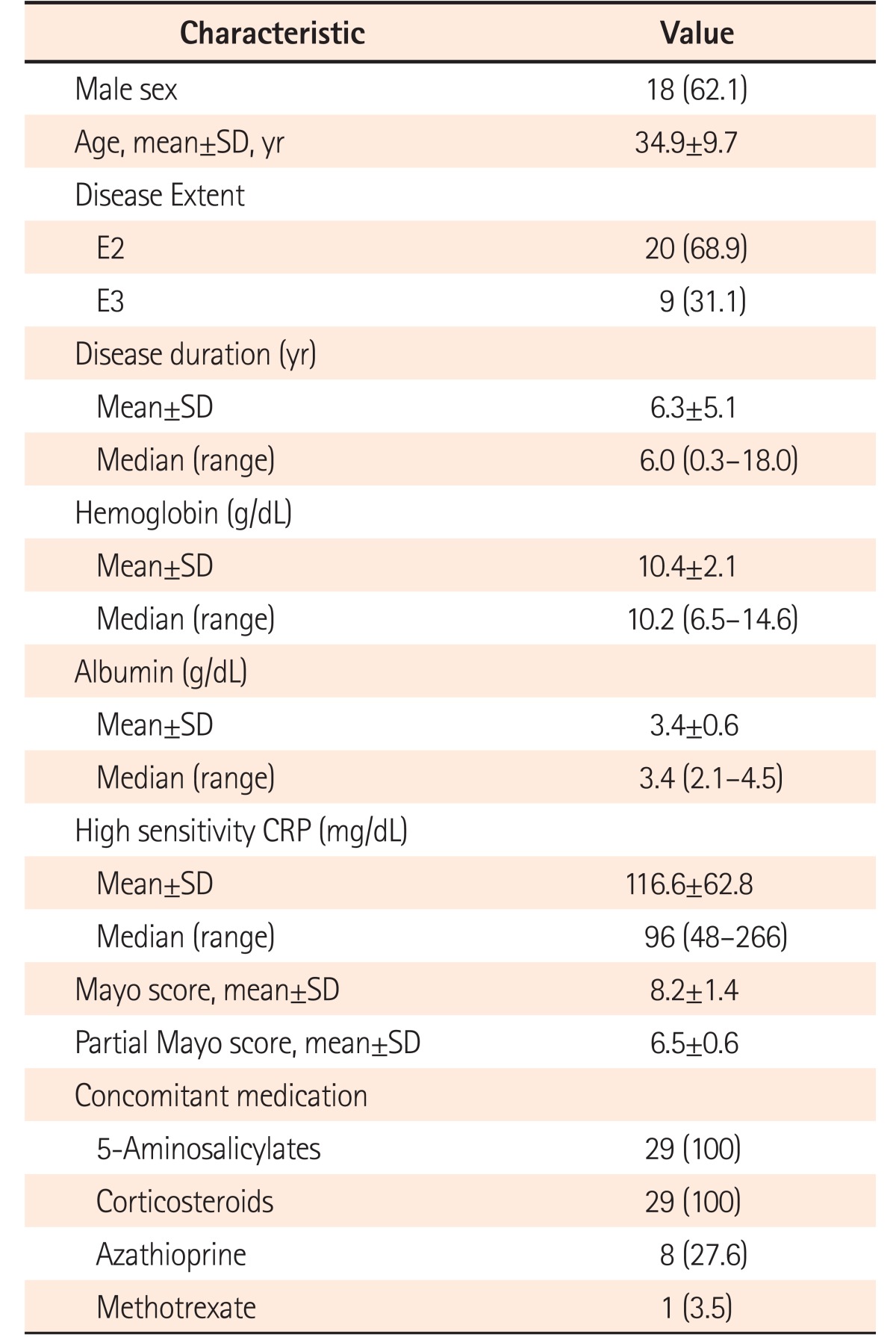
A total of 29 patients (62.1% male; mean age, 34.9 ± 9.7 years) with moderate-to-severe steroid-refractory active UC (mean disease duration, 6.3±5.1 years; pancolitis in 9 patients [31.1%]; left-sided colitis in 20 patients [68.9%]) were treated with the Exemptia induction dosing schedule. The mean Mayo score at presentation was 8.2±1.4. At week 8, clinical response was observed in 7 patients (24.1%), whereas clinical remission was observed only in 1 patient (3.5%). Among the non-responders (n=21), 4 patients required colectomy, 1 died, 1 was lost to follow-up, 10 were offered fecal microbiota transplant, 3 were administered infliximab, and 2 patients were administered cyclosporine and tacrolimus, respectively. Four patients (13.8%) developed extrapulmonary tuberculosis.
Ulcerative colitis (UC) is an IBD, which can progress over time to extensively involve the colon and significantly affect health and related quality of life.1 The disease severity may be high in up to 15% to 20% of patients, who require hospitalization and treatment with corticosteroids.2 In patients who fail to respond to 3–5 days of intravenous corticosteroids and optimal supportive care, the available therapeutic options include the use of intravenous cyclosporine, anti-tumor necrosis factor-α (anti-TNF-α) agents, or total colectomy.
TNF-α is a pro-inflammatory cytokine that plays an important role in both the initiation of the disease process in IBD and in the amplification of the inflammatory cascade.3 Antibodies against TNF-α have been used in novel rescue therapies for patients with moderate-to-severe acute UC. The first of these agents, infliximab, was shown to be safe and effective in randomized controlled trials in patients with severe UC and decreased the risk of colectomy at 1 year.4 Adalimumab, a fully human recombinant monoclonal IgG1 antibody, which binds with high affinity to TNF-α and inhibits the binding of TNF-α with its receptor, was the 2nd biological agent to be evaluated for use in moderate-to-severe acute UC. The initial uncontrolled experience with adalimumab in UC was disappointing.5 However, after the Ulcerative Colitis Long Term Remission and Maintenance with Adalimumab (ULTRA) 1 and ULTRA 2 trials established the safety and efficacy of adalimumab in moderate-to-severe UC, it was approved for use by the Food and Drug Administration in 2012.678 Since these 2 randomized placebo-controlled trials, a few “real-world” studies have also reinforced the efficacy of adalimumab in moderate-to-severe active UC.5910111213141516
The costs of these biological drugs are high and as the patents for these drugs have expired, interest in biosimilars has emerged. Biosimilars, according to the World Health Organization (WHO), are biotherapeutic products with similar quality, safety, and efficacy to already-licensed reference biotherapeutic products.17 In developing countries, such as India, where the use of biologicals has been limited by the high costs that must be borne by patients, the introduction of biosimilars has been a welcome change. Although there have been many studies on the safety and efficacy of the infliximab biosimilar CT-P13 (Remsima, Celltrion, Incheon, Korea; Inflectra, Hospira, Lake Forest, IL, USA) in IBD, the data on the biosimilars of other drugs in IBD are sparse.181920212223 In 2014, the first adalimumab biosimilar drug, Exemptia (ZRC-3197), was commercialized by Zydus Cadila (Ahmedabad, India).24 Exemptia is a fully human monoclonal IgG1 antibody produced recombinantly from Chinese hamster ovary cells. It is a 1330-residue amino acid that contains a glycoprotein with 2 copies of heavy‐chains and 2 copies of light‐chains in the heterodimeric form with a molecular weight of approximately 148 kDa.25
Here, we have reported the first “real-life” experience with the adalimumab biosimilar Exemptia in patients with moderate-to-severe active UC refractory to steroids.
This was a retrospective observational study to evaluate the treatment outcomes of Exemptia, an adalimumab biosimilar, in patients with moderate-to-severe UC who were refractory to steroid treatment. The study was conducted between June 2015 and December 2016. The study was approved by the Institutional Review Board of Dayanand Medical College and Hospital, Ludhiana, India (IRB No. 2017-274) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
The diagnosis of UC was established by colonoscopy/flexible sigmoidoscopy and histopathology at the start of treatment. All patients with moderate-to-severe active UC, as defined by a Mayo score of 6 to 12 points (with an endoscopic subscore of ≥2), who were refractory to treatment with steroids (lack of clinical response to glucocorticoids after 5–7 days of intravenous hydrocortisone 100 mg every 8 hours) were offered biologics (infliximab/adalimumab) or biosimilars, cyclosporine, or surgery. The patients who were treated with the adalimumab biosimilar were enrolled in the present study. Patients with toxic megacolon, ulcerative proctitis, indeterminate colitis/Crohn's colitis, and current bacterial and viral infections (hepatitis B, hepatitis C, or human immunodeficiency virus) were excluded. Clostridium difficile was excluded by testing the stool samples for glutamate dehydrogenase and for C. difficile toxin A/B.
All patients were screened for tuberculosis with a detailed history (suggestive of disease in the patient and the history of present or past exposure to patient with tuberculosis) and physical examination and chest radiograph to exclude lesions suggestive of pulmonary tuberculosis. Latent tuberculosis was excluded by tuberculin skin testing and interferon-γ release assays (QuantiFERON TB gold). Infection with cytomegalovirus was excluded by blood tests for IgM cytomegalovirus (CMV) and/or CMV DNA and the histopathological examination of rectal biopsy (H&E stain was used to rule out owl's eye inclusion bodies). Patients with demyelinating diseases of the CNS and malignancies were also excluded. Female of childbearing age underwent a urine pregnancy test at screening and were advised to use contraception throughout the study period.
Eligible patients received a total of 5 SC injections of Exemptia; 160 mg at week 0, 80 mg at week 2, and then 40 mg every other week from week 4 to week 8. The dose of concomitant medications, such as 5-aminosalicylate, remained constant, and the corticosteroids were tapered slowly over 2 to 3 months. After induction with Exemptia, the patients subsequently administered a maintenance dose of azathioprine.
The patients were evaluated at weeks 0, 2, 4, and 8. A Mayo score was determined at weeks 0 and 8. At 2 and 4 weeks, a partial Mayo score (excluding endoscopic findings) was determined. The clinical response and clinical remission were determined at week 8. A repeat colonoscopy was also performed at week 8 to assess mucosal healing. Clinical remission was defined as a total Mayo score of ≤2 points, with no individual score >1 point. Clinical response was defined as decrease in the total Mayo score by at least 3 points or >30% from the baseline, with a decrease in rectal bleeding score by at least 1 point and an absolute rectal bleeding score of 0 or 1. Mucosal healing was defined as an endoscopic subscore of ≤1.
At the start of therapy, the patients were monitored daily for the number of bowel movements (day and night), the presence of blood in their stools, abdominal pain, or cramping, and vital signs (blood pressure and heart rate) were monitored every 6 hours. Complete blood count and electrolytes were checked daily, and serum albumin, ESR, and CRP were evaluated every 3rd day when the patients were at the hospital. The patients who were discharged were followed up every 2 weeks with complete blood count, ESR, CRP, and albumin. Adverse events, if any, were recorded.
The baseline demographics and clinical characteristics are summarized in Table 1. The adalimumab biosimilar Exemptia was used as a salvage therapy in a total of 29 patients with moderate-to-severe active UC who did not respond to a 5-day course of intravenous steroids. The majority of patients were male (62.1%), and the average age of the patients at presentation was 34.9±9.7 years. The average duration of the disease was 6.3±5.1 years. More than two-thirds of the patients (n=20, 68.9%) had left-sided disease, whereas 31.1% had pancolitis. Eight patients were receiving azathioprine and 1 was receiving methotrexate prior to the administration of adalimumab.
The mean Mayo score at presentation was 8.2±1.4 (range, 6–12; moderate disease [Mayo score 6–10], 27 patients; severe disease [Mayo score 11–12], 2 patients).
At week 8, clinical response was observed in 7 patients (24.1%), but clinical remission was observed only in 1 patient (3.5%) (Fig. 1). Adverse events during treatment were noted in 4 patients: 2 of them developed pulmonary tuberculosis and the other 2 had tubercular pericardial effusion, 1 of whom succumbed to cardiac arrhythmias. Injection site reactions were noted in 2 patients; these were mild and managed conservatively. None of the patients exhibited leucopenia or demyelination.
As shown in Fig. 2, the responders (n=8, 27.6%) were maintained on azathioprine after the initial induction treatment with Exemptia. However, a majority of patients (n=28) did not achieve clinical remission. Among the 21 patients who did not respond, 4 required colectomy (2 required colectomy after 2 doses of adalimumab). There was 1 death, and 1 patient was lost to follow-up. Fecal microbiota transplantation was offered to 10 of the remaining non-responders. Other pharmacological salvage measures included infliximab (n=3), cyclosporine (n=1), and tacrolimus (n=1).
The adalimumab biosimilar Exemptia displayed a limited efficacy for the induction of remission in our patients with moderate-to-severe active UC. Despite an initial clinical response in 7 patients (24.1%), only 1 patient (3.5%) achieved clinical remission. Four patients (13.8%) required colectomy and 4 developed tuberculosis; 1 of whom succumbed to a cardiac ailment.
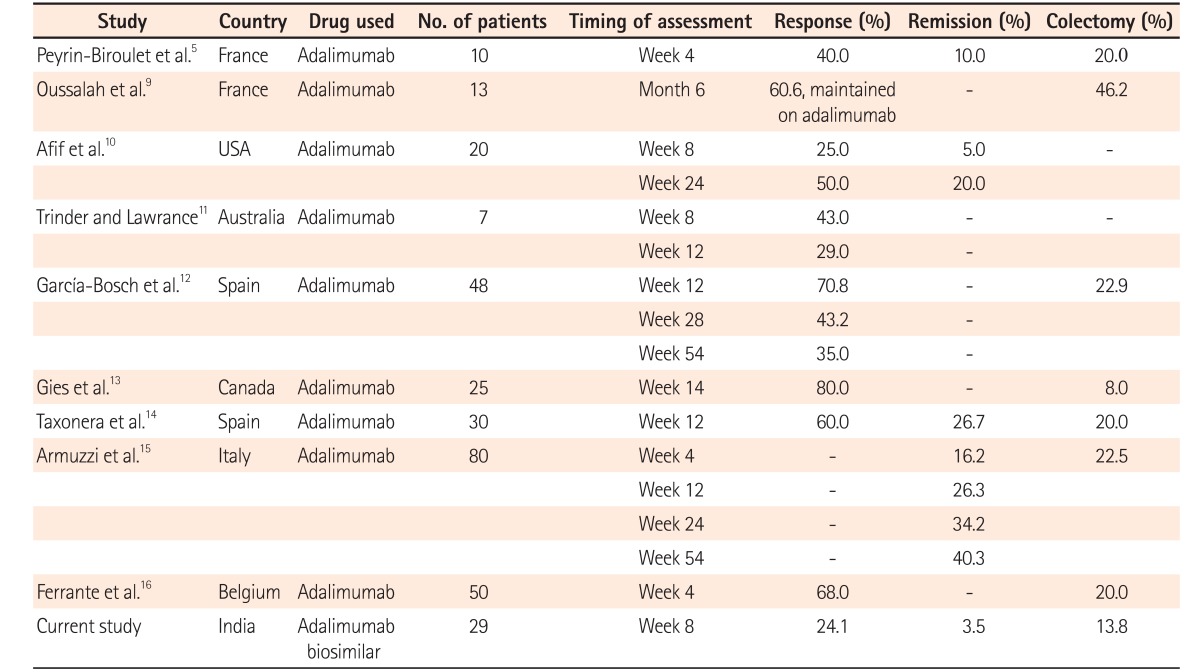
Adalimumab has been approved worldwide for the treatment of moderate-to-severe active UC. The safety and efficacy of adalimumab for use in moderate-to-severe active UC was first established by 2 multicenter, double-blind, randomized placebo-controlled trials—ULTRA 1 and ULTRA 2.67 The ULTRA1 trial included infliximab-naïve patients, and clinical remission was noted in 18.5% of patients who were administered adalimumab 160 mg at week 0, 80 mg at week 2, and 40 mg at weeks 4 and 6, which was twice as high as that in the placebo group (9.2%). Clinical response and an improvement in Mayo subscore were also observed in a higher proportion of patients administered adalimumab. The ULTRA2 trial included patients who had previously received infliximab in the past. At week 8, a significantly higher number of the 248 patients administered adalimumab (160 mg at week 0, 80 mg at week 2, and then 40 mg every other week beginning at week 4) showed clinical remission (16.5% vs. 9.3% on placebo, P =0.019) and clinical response (50.4% vs. 34.6% on placebo, P <0.001). However, in the subgroup analysis, adalimumab was effective only in patients who were naïve to anti-TNF-α therapy. Several real-world experiences of adalimumab have subsequently reinforced the safety and efficacy of adalimumab in UC, especially in patients with intolerance or loss of response to infliximab (Table 2).5910111213141516 At present, to the best of our knowledge, no real-world experience of Exemptia have been reported.
In contrast with these randomized controlled trials and real-world data of adalimumab, Exemptia was not very efficacious for the management of steroid-resistant moderate-to-severe UC in our patients (Table 2). In comparison with studies from the West, in which mucosal healing was achieved in more than 40% of the patients, the rate of mucosal healing (3.5%) in our study was poor.7 A possible explanation for this was that the Exemptia dose used was the same as that of adalimumab in the ULTRA 1 trial, which was subsequently based on the effective doses in CD.6 In the subgroup analysis of the ULTRA 1 trial, patients with elevated CRP were found to have lower rates of clinical remission. As our patients had a higher inflammatory burden, as evidenced by the very high CRP values (range, 48–266 with median 96 mg/dL), a higher dose of Exemptia may have beenbeneficial in such patients.
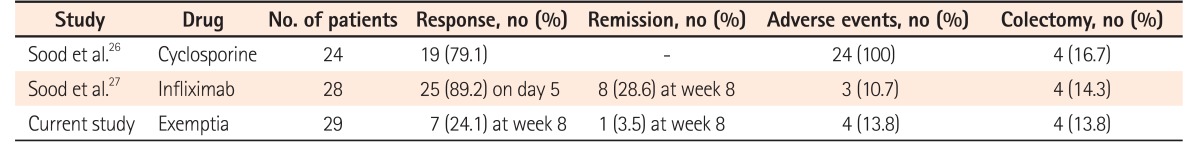
As the design and characteristics of our study population may differ from those in the Western world, we compared the results of our experiences with cyclosporine A and infliximab as salvage therapies in patients with acute severe UC (Table 3), and found lower response rates to Exemptia than to therapies.2627
In addition to a lower efficacy, a larger number of clinically significant side effects were observed in our cohort of patients than inpatients of Western studies. Four patients (13.8%) developed tuberculosis, which was extrapulmonary in 2 cases, and 1 patient succumbed to tubercular pericardial effusion. In developing countries such as India, where 40% of the population is exposed to tuberculosis (a majority of whom have latent tuberculosis), biologicals and biosimilars should be prescribed with caution, and screening for latent tuberculosis must be conducted before the start of treatment.28
Our study has a few limitations: the number of patients treated with Exemptia was small, and, owing to financial limitations, Exemptia was used only for the induction of remission and not for maintenance. Adalimumab is not available in India; hence, the efficacy of Exemptia was compared with the data on adalimumab from other countries.
In conclusion, the adalimumab biosimilar Exemptia has limited efficacy when used for induction of remission in moderate-to-severe steroid-refractory UC. However, large, multicenter trials that include all patients with active UC are required to allow a discussion of the role of Exemptia in the overall medical management of UC.
Notes
Financial support: The authors received no financial support for the research, authorship, and/or publication of this article.
AUTHOR CONTRIBUTION: Conception and design; collection, analysis and interpretation of the data; drafting of the article; critical revision of the article for important intellectual content; final approval of the article : Vandana Midha, R.M., and Ajit Sood.
Critical revision of the article for important intellectual content; final approval of the article: Varun Mehta, V.N., Arshdeep Singh, and K.K.
References
1. Rubin DT, Siegel CA, Kane SV, et al. Impact of ulcerative colitis from patients' and physicians'perspectives: results from the UC. NORMAL survey. Inflamm Bowel Dis. 2009; 15:581–588. PMID: 19067414.
2. Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996; 38:905–910. PMID: 8984031.

3. Danese S, Angelucci E, Malesci A, Caprilli R. Biological agents for ulcerative colitis: hypes and hopes. Med Res Rev. 2008; 28:201–218. PMID: 17464967.

4. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476. PMID: 16339095.

5. Peyrin-Biroulet L, Laclotte C, Roblin X, Bigard MA. Adalimumab induction therapy for ulcerative colitis with intolerance or lost response to infliximab: an open-label study. World J Gastroenterol. 2007; 13:2328–2332. PMID: 17511032.

6. Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011; 60:780–787. PMID: 21209123.

7. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012; 142:257–265.e3. PMID: 22062358.

8. Humira (adalimumab). Food and Drug Administration. April 23, 2017. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/125057orig1s232ltr.pdf.
9. Oussalah A, Laclotte C, Chevaux JB, et al. Long-term outcome of adalimumab therapy for ulcerative colitis with intolerance or lost response to infliximab: a single-centre experience. Aliment Pharmacol Ther. 2008; 28:966–972. PMID: 18652603.

10. Afif W, Leighton JA, Hanauer SB, et al. Open-label study of adalimumab in patients with ulcerative colitis including those with prior loss of response or intolerance to infliximab. Inflamm Bowel Dis. 2009; 15:1302–1307. PMID: 19408340.

11. Trinder MW, Lawrance IC. Efficacy of adalimumab for the management of inflammatory bowel disease in the clinical setting. J Gastroenterol Hepatol. 2009; 24:1252–1257. PMID: 19220669.

12. García-Bosch O, Gisbert JP, Cañas-Ventura A, et al. Observational study on the efficacy of adalimumab for the treatment of ulcerative colitis and predictors of outcome. J Crohns Colitis. 2013; 7:717–722. PMID: 23142005.

13. Gies N, Kroeker KI, Wong K, Fedorak RN. Treatment of ulcerative colitis with adalimumab or infliximab: long-term follow-up of a single-centre cohort. Aliment Pharmacol Ther. 2010; 32:522–528. PMID: 20500733.

14. Taxonera C, Estellés J, Fernández-Blanco I, et al. Adalimumab induction and maintenance therapy for patients with ulcerative colitis previously treated with infliximab. Aliment Pharmacol Ther. 2011; 33:340–348. PMID: 21133961.

15. Armuzzi A, Biancone L, Daperno M, et al. P230 Adalimumab in active ulcerative colitis: a “real-life” observational study. J Crohns Colitis. 2012; 6(Suppl 1):S101–S101.

16. Ferrante M, Karmiris K, Compernolle G, Ballet V, Vermeire S, Noman M. Efficacy of adalimumab in patients with ulcerative colitis: restoration of serum levels after dose escalation results in a better long-term outcome. Gut. 2011; 60(Suppl 3):A72.
17. Guidelines on evaluation of similar biotherapeutic products (SBPs). World Health Organization;Accessed May 4, 2017. http://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf.
18. Park SH, Kim YH, Lee JH, et al. Post-marketing study of biosimilar infliximab (CT-P13) to evaluate its safety and efficacy in Korea. Expert Rev Gastroenterol Hepatol. 2015; 9(Suppl 1):35–44. PMID: 26395533.

19. Jung YS, Park DI, Kim YH, et al. Efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol. 2015; 30:1705–1712. PMID: 25974251.

20. Kang YS, Moon HH, Lee SE, Lim YJ, Kang HW. Clinical experience of the use of CT-P13, a biosimilar to infliximab in patients with inflammatory bowel disease: a case series. Dig Dis Sci. 2015; 60:951–956. PMID: 25326115.

21. Gecse KB, Lovász BD, Farkas K, et al. Efficacy and safety of the biosimilar infliximab CT-P13 treatment in inflammatory bowel diseases: a prospective, multicentre, nationwide cohort. J Crohns Colitis. 2016; 10:133–140. PMID: 26661272.

22. Farkas K, Rutka M, Bálint A, et al. Efficacy of the new infliximab biosimilar CT-P13 induction therapy in Crohn's disease and ulcerative colitis: experiences from a single center. Expert Opin Biol Ther. 2015; 15:1257–1262. PMID: 26134250.

23. Park DI. Current status of biosimilars in the treatment of inflammatory bowel diseases. Intest Res. 2016; 14:15–20. PMID: 26884730.

24. Adalimumab biosimilar. autoimmune disorders. rheumatoid arthritis. juvenile idiopathic arthritis. psoriatic arthritis, ankylosing spondylitis. Exemptia;Accessed May 4, 2017. https://exemptia.com/zydus-launches-worlds-first-biosimilar-of-adalimumab-2/.
25. Jani RH, Gupta R, Bhatia G, et al. A prospective, randomized, double-blind, multicentre, parallel-group, active controlled study to compare efficacy and safety of biosimilar adalimumab (Exemptia; ZRC-3197) and adalimumab (Humira) in patients with rheumatoid arthritis. Int J Rheum Dis. 2016; 19:1157–1168. PMID: 26176644.

26. Sood A, Midha V, Sood N, et al. Cyclosporine in the treatment of severe steroid refractory ulcerative colitis: a retrospective analy-sis of 24 cases. Indian J Gastroenterol. 2008; 27:232–235. PMID: 19405256.
27. Sood A, Midha V, Sharma S, et al. Infliximab in patients with severe steroid-refractory ulcerative colitis: Indian experience. Indian J Gastroenterol. 2014; 33:31–34. PMID: 23999683.

28. TB statistics for India: national and state statistics. TB Facts.org;April 26, 2017. http://www.tbfacts.org/tb-statistics-india/.
Fig. 1
Efficacy of Exemptia in treating moderate-to-severe active UC (total=29). Among the 29 patients with moderate-to-severe UC who were treated with Exemptia, clinical response at week 8 was observed in 7 patients (24.1%), but only 1 patient (3.5%) achieved clinical remission.
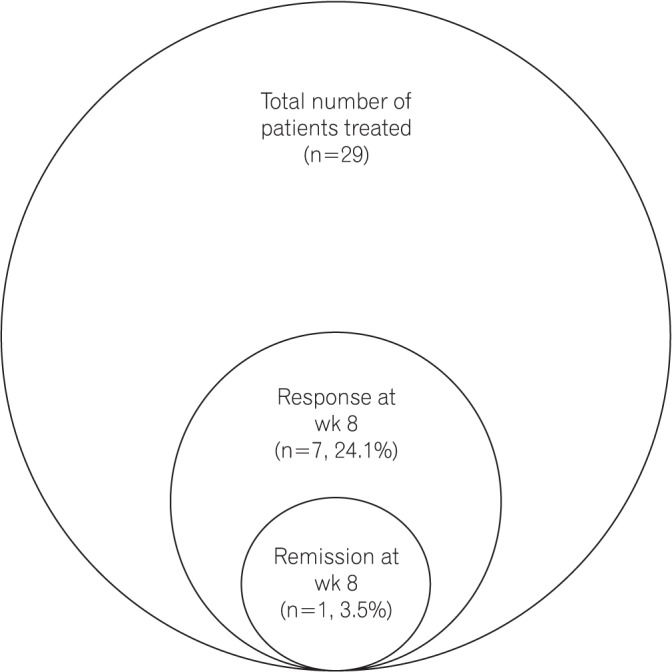
Fig. 2
Response/remission in patients with moderate-to-severe active UC treated with Exemptia. Patients with moderate-to-severe active UC who showed clinical response at week 8 (n=8, 27.6%) were maintained on azathioprine after the initial induction therapy with Exemptia. Only 1 of 7 patients achieved clinical remission at week 8. Of the 21 patients who did not respond, 4 required colectomies, 1 died, and 1 was lost to follow-up. Salvage measures in the other patients included fecal microbiota transplantation (n=10), infliximab (n=3), cyclosporine (n=1), and tacrolimus (n=1) administration.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download