Abstract
Background/Aims
Colonoscopic surveillance is currently recommended after polypectomy owing to the risk of newly developed colonic neoplasia. However, few studies have investigated colonoscopy surveillance in Asia. This multicenter and prospective study was undertaken to assess the incidence of advanced adenoma based on baseline adenoma findings at 3 years after colonoscopic polypectomy.
Methods
A total of 1,323 patients undergoing colonoscopic polypectomy were prospectively assigned to 3-year colonoscopy surveillance at 11 tertiary endoscopic centers. Relative risks for advanced adenoma after 3 years were calculated according to baseline adenoma characteristics.
Results
Among 1,323 patients enrolled, 387 patients (29.3%) were followed up, and the mean follow-up interval was 31.0±9.8 months. The percentage of patients with advanced adenoma on baseline colonoscopy was higher in the surveillance group compared to the non-surveillance group (34.4% vs. 25.7%). Advanced adenoma recurrence was observed in 17 patients (4.4%) at follow-up. The risk of advanced adenoma recurrence was 2 times greater in patients with baseline advanced adenoma than in those with baseline non-advanced adenoma, though the difference was not statistically significant (6.8% [9/133] vs. 3.1% [8/254], P=0.09). Advanced adenoma recurrence was observed only in males and in subjects aged ≥50 years. In contrast, adenoma recurrence was observed in 187 patients (48.3%) at follow-up. Male sex, older age (≥50 years), and multiple adenomas (≥3) at baseline were independent risk factors for adenoma recurrence.
Removal of colorectal adenomas during colonoscopy has been shown to reduce the risk of future colorectal cancer (CRC) and advanced adenomas. To further minimize the risk of CRC, surveillance with colonoscopy is recommended after the detection and removal of colonic neoplasia. In the past, it was common practice to perform annual follow-up surveillance colonoscopies in patients with colorectal adenomas in order to detect newly developed metachronous adenomas and missed synchronous adenomas.1 However, most patients with colorectal adenoma experience no benefit from surveillance and only 6% develop CRC,2 suggesting that, ideally, surveillance should be targeted at patients most likely to develop CRC. Several studies have found that certain adenoma characteristics at baseline colonoscopy are associated with the rate of adenoma detection and the histologic severity of subsequent adenomas during surveillance.3
4 Patients with multiple adenomas or adenomas with advanced pathological features at baseline have been found to have an increased risk of developing CRC.4
5
Current Western guidelines have recommended colonoscopy surveillance intervals according to the risk stratification of patients based on the findings at baseline colonoscopy.6 According to U.S. guidelines, surveillance colonoscopy should be repeated at 10 years for patients with no neoplasia at baseline, at 5 to 10 years for low-risk patients with 1 or 2 small (<10 mm) tubular adenomas, and at 3 years for high-risk patients with advanced neoplasia or more than 2 adenomas.7 However, to date, few studies have evaluated colonoscopy surveillance after polypectomy in Asia.8
The aims of this prospective and multicenter study were to determine the 3-year cumulative incidence rate of advanced neoplasia in Korean patients with baseline colorectal neoplasia at screening colonoscopy and to determine which baseline colonoscopic findings are associated with the recurrence of advanced neoplasia.
Participants were enrolled in 11 University Hospitals between March 2007 and December 2008. The study protocol was approved by the Institutional Review Boards of each participating center. Written informed consent for participation was obtained from all participants prior to the procedures. Initial enrollment criteria included individuals who did not have lower gastrointestinal tract symptoms, prior history of colon disease, or structural examination of the colon within 10 years. Patients were eligible if they underwent a complete colonoscopy and were found to have 1 or more adenomas at baseline colonoscopy. A complete colonoscopy was considered to include the following: good bowel preparation, colonoscopy reaching the cecum, and removal of all visualized polyps that were detected. Patients were excluded if baseline colonoscopy revealed invasive CRC (cancer invading beyond muscularis mucosa), nonadenomatous polyps, IBD, or familial adenomatous polyposis.
At enrollment, patients were interviewed to assess the following data: age; gender; family history of CRC; current smoking and alcohol consumption; history of diabetes and hypertension; use of medications including aspirin, NSAIDs, and statins.
All enrolled patients were assigned to colonoscopy surveillance at 3 years. Follow-up clinic reminder calls were made several months before the expected date of colonoscopy surveillance from each participating center.
All colonoscopic examinations were performed by study investigators. During baseline and surveillance colonoscopy, the characteristics of all detected polyps including number, size, and location were identified. The size of the polyp was estimated with an opened biopsy forceps. The location of the polyp was categorized as proximal (cecum, ascending colon, hepatic flexure, transverse colon, and splenic flexure) or distal (descending colon, sigmoid colon, and rectum). All detected polyps were completely removed; diminutive polyps were removed by ≤5 mm by biopsy forceps and larger ones by colonoscopic polypectomy or endoscopic mucosal resection. All specimens were evaluated by local expert gastrointestinal pathologists according to the colorectal neoplasia classification of the World Health Organization (WHO) recommendations.9
Pathological interpretation of intramucosal carcinoma or carcinoma in situ was categorized as a high-grade dysplasia. Invasive cancer was defined as the invasion of malignant cells beyond the muscularis mucosa. In patients with multiple adenomas, the size, location, and histological type were classified according to the largest, the most proximal, and the most advanced lesion, respectively. Advanced adenoma was defined as tubular adenoma 10 mm or larger in diameter, villous or tubulovillous adenoma, or adenoma with high-grade dysplasia.
We compared the baseline demographic and adenoma characteristics of subjects who underwent follow-up colonoscopy with those of subjects who did not using the Student t-test and chi-square test as appropriate for the comparison of means and proportions. The RR of recurrence of advanced adenoma and any adenoma were estimated using the Cox proportional hazard regression model by computing the hazard ratio and the 95% CI after multivariate adjustments (demographic and baseline adenoma characteristics). Statistical analyses were performed using SPSS version 12.0 for Windows (SPSS Inc, Chicago, IL, USA). A two-sided value of P<0.05 was considered statistically significant.
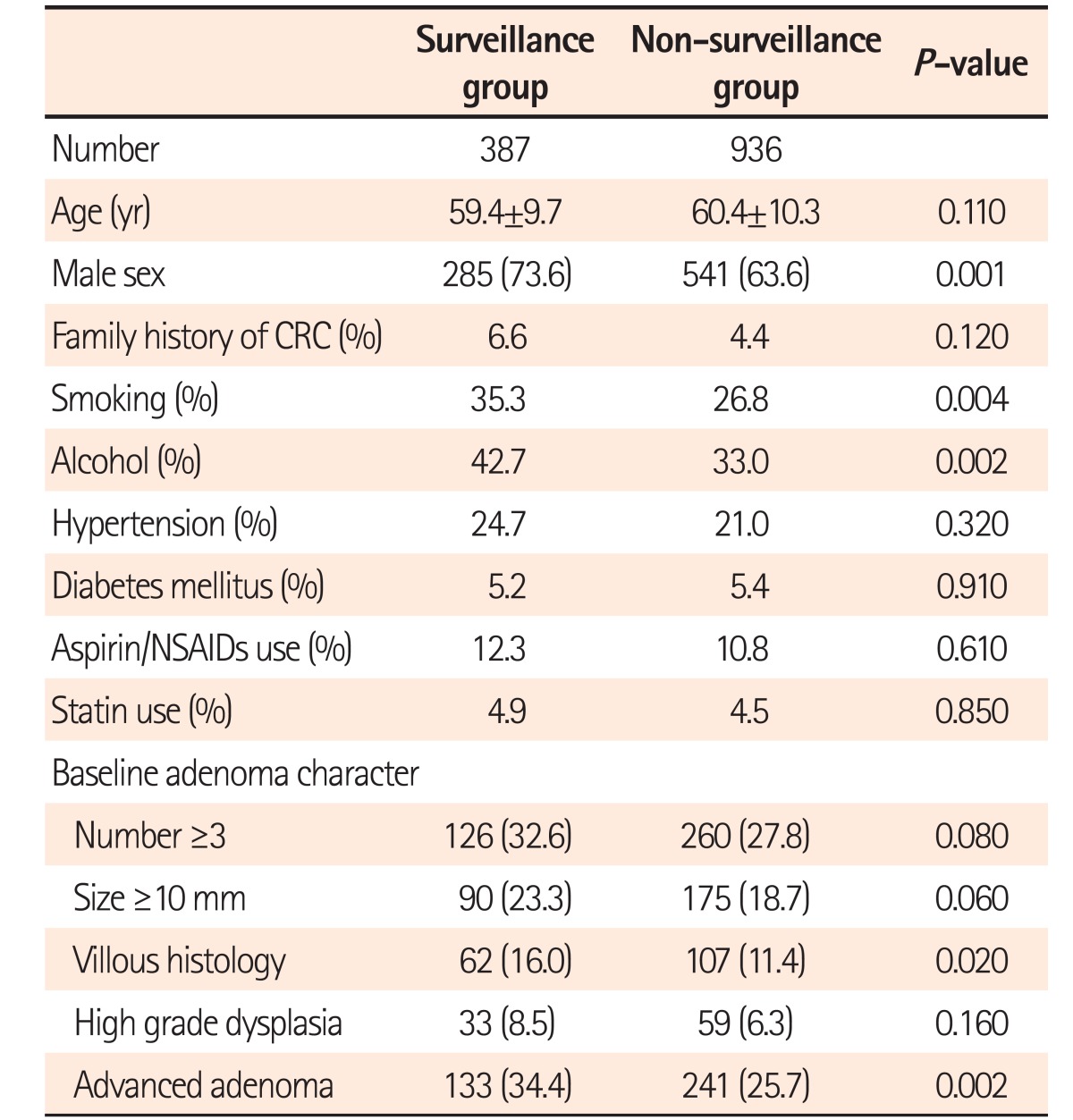
A total of 1,323 patients with colorectal adenoma at baseline colonoscopy were enrolled and assigned to colonoscopy surveillance at 3 years. During surveillance, 29.3% of patients (387/1,323) had a surveillance colonoscopic examination, and the mean follow-up interval was 31.0±9.8 months. We compared the baseline demographic and adenoma characteristics of the surveillance group with those of the non-surveillance group to determine whether there were any differences in the possible risk factors for advanced colorectal neoplasia between the 2 groups (Table 1). There were no significant differences in terms of age, family history of CRC, co-morbidities (hypertension and diabetes), and use of medications (aspirin, NSAIDs, and statins). However, the surveillance group had higher proportions of males, smokers, and alcohol drinkers, and was more likely to have purported high CRC risk variables of baseline adenoma including advanced adenoma, number, size, and villous histology. Accordingly, we analyzed the incidence and risk factors of colorectal adenoma recurrence in the surveillance group.
During the follow-up period of 31.0±9.8 months, the recurrence rates of advanced adenoma, multiple adenoma with 3 or more, and any adenoma were 4.4% (17/387), 10.6% (41/387), and 48.3% (187/387), respectively.
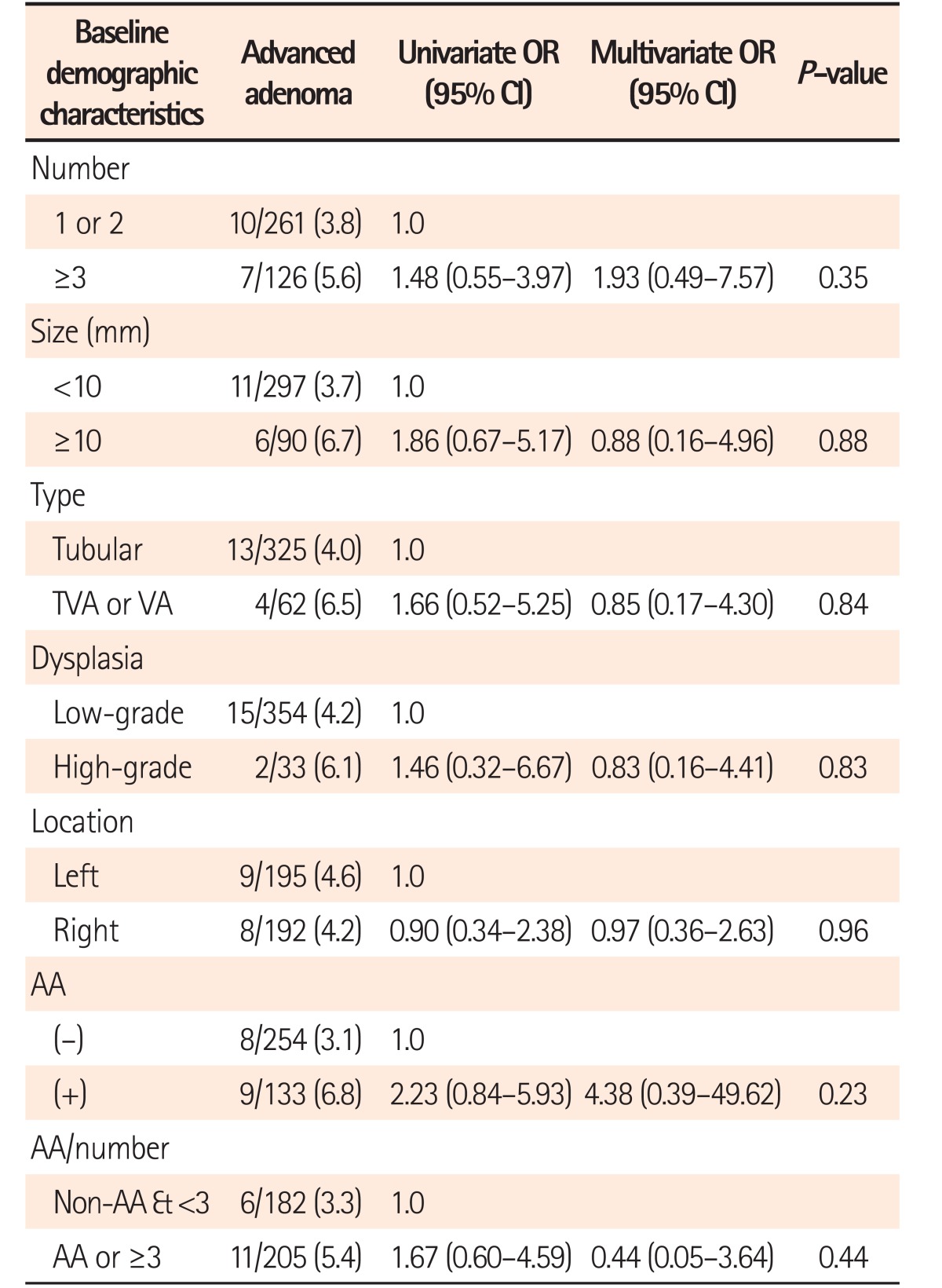
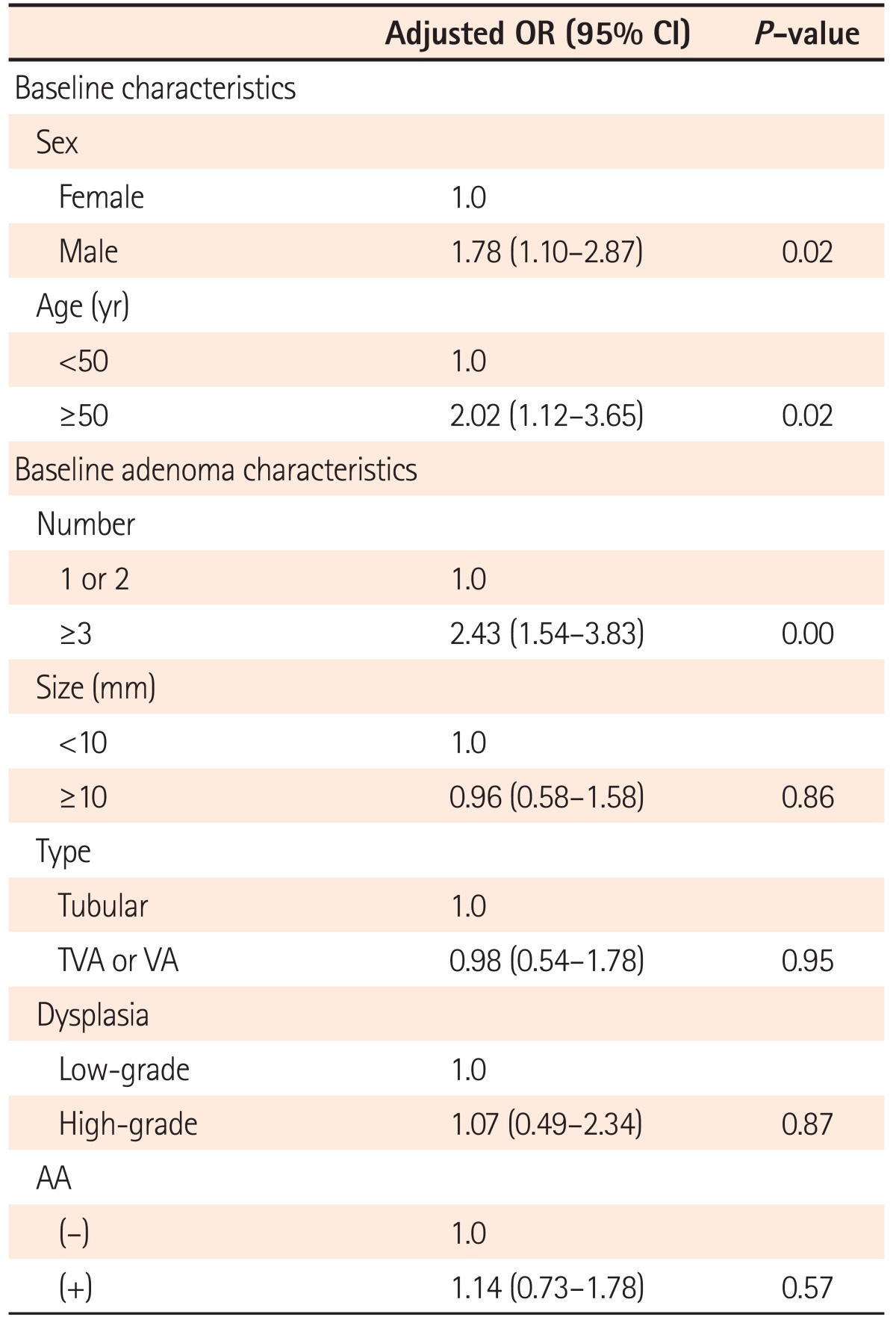
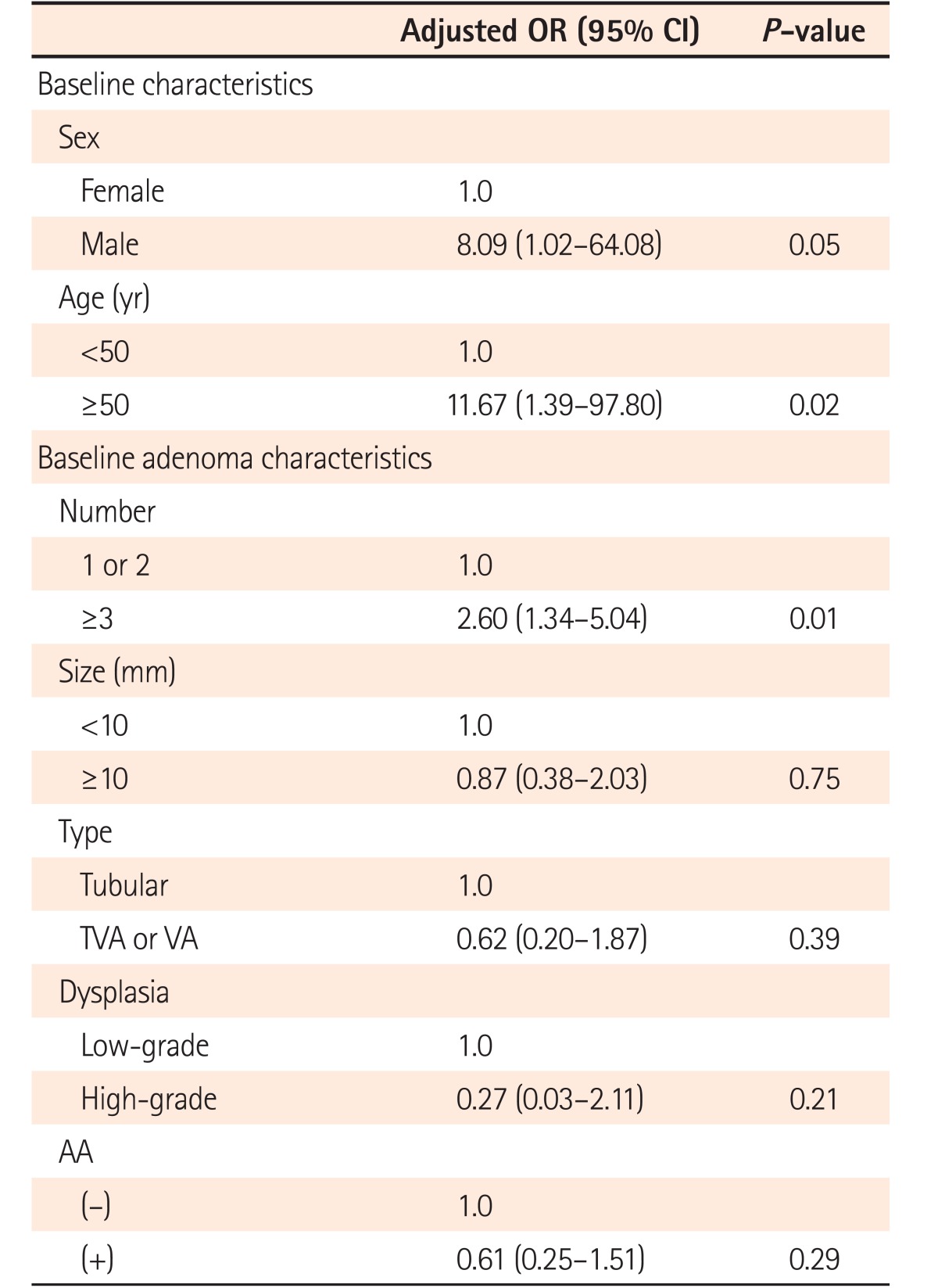
Tables 2 and 3 demonstrate the RR of demographic and baseline adenoma characteristics for recurrent advanced adenoma after multivariate adjustments. Among the demographic variables, advanced adenoma recurrence was observed only in males and in subjects aged ≥50 years at follow-up (Table 2). On the contrary, there was no significant risk factor for advanced adenoma recurrence among the baseline adenoma variables. However, the risk of advanced adenoma recurrence was 2 times higher in patients with baseline advanced adenoma than in patients with baseline non-advanced adenoma, though the difference was not statistically significant (6.8% [9/133] vs. 3.1% [8/254], P=0.09] (Table 3).
Tables 4 and 5 present the RR associated with each of the demographic and baseline adenoma characteristics for recurrent overall colorectal adenoma and multiple (≥3) adenomas, respectively. Male sex, age ≥50 years, and number of adenomas (≥3) had a significant impact on both overall adenoma recurrence and multiple (≥3) colorectal adenoma recurrence after multivariate adjustments.
In the present multicenter, prospective study, the 3-year cumulative incidence rate of advanced adenoma in Korean patients with colorectal adenoma at the baseline screening colonoscopy was 4.4%. The risk of advanced adenoma recurrence was 2 times higher in patients with baseline advanced adenoma than in patients with baseline non-advanced adenoma. Advanced adenoma recurrence was observed only in males and subjects aged ≥50 years at follow-up. In addition, male sex, older age (≥50 years), and multiple adenomas (≥3) were independent risk factors for overall adenoma and multiple adenoma recurrence.
According to the official reports of Korea Central Cancer Registry, the incidence rates for CRC have continued to increase over the past 14 years (1999–2012) in Korea because of the spread of the Westernized lifestyle, particularly in terms of dietary habits.10
11 CRC was the second most common cancer in males, the third most common in females, and the fourth cause of cancer deaths in 2012 in Korea.12
13 Screening colonoscopy and polypectomy are important to reduce the incidence and mortality of CRC.14 Patients with removed adenomas are advised to have follow-up surveillance colonoscopy according to risk stratification based on the findings at baseline colonoscopy. However, few studies have been conducted on follow-up surveillance colonoscopy after polypectomy in Asia.
Patients with baseline adenoma are considered at higher risk of colorectal neoplasia, even after their polyps have been removed.4
15
16
17 Recurrent adenomas of any kind were found in about 20% to 49% of patients who underwent follow-up colonoscopy within 3 to 5 years after polypectomy in several Western population-based studies,15
18
19
20
21
22 and advanced adenomas were detected in 6% to 13% of these patients. In a Chinese population-based study, the cumulative recurrence rates of any adenoma and of advanced adenoma were 21.1% and 2.2%, respectively, during the surveillance interval of 1 to 3 years after polypectomy.23 One Japanese multicenter retrospective study has reported the 3-year cumulative incidence of large adenomatous polyps ≥10 mm, intramucosal cancer, and invasive cancer to be 3.9% after polypectomy.24 In Korea, a prospective study has reported recurrent adenoma rates of about 33.3% within 3 years after polypectomy, with about 6.6% of recurrences being advanced adenoma.3 In the present study, the 3-year recurrence rates of any adenoma (48.3%) and advanced adenoma (4.4%) were similar to those studies.
We also investigated the risk factors for adenoma recurrence in all patients who underwent surveillance colonoscopy. Although a few studies have supported the effects of age,15
21
25 gender,22
24
26 family history,27
28 obesity,29
30 smoking,31
32 and aspirin/NSAID33 or statin use34 on adenomas, the issue remains controversial. The Asia Pacific Working Group reported that advancing age, male sex, family history, and smoking are the most important risk factors for developing advanced CRC.13 A report from a pooled analysis of postpolypectomy patients showed that older age and male sex were associated with an increased risk of metachronous advanced adenoma.21 One Japanese study24 and one Chinese study23 also reported that the occurrence of advanced neoplasia at surveillance colonoscopy was associated with the male sex and older age, while another Chinese study25 reported that older age alone was an important risk factor for advanced neoplasia recurrence. Several previous studies suggested that males have a higher prevalence of advanced adenoma than females during surveillance colonoscopy.22
26 In this prospective study, we confirmed that the male sex and age ≥50 years had a significant effect on the recurrence of any adenoma, multiple adenomas, and advanced adenoma. In the multivariate analysis, family history, smoking, and medication were not associated with advanced adenoma recurrence. However, the low rate of patients who underwent surveillance colonoscopy and the low incidence of advanced adenoma on follow-up may have contributed to the negative results.
A number of studies have reported that the size, number, and histological characteristics of the baseline adenoma were associated with an increased risk of advanced adenoma recurrence.4
21
25
27
35 Some studies have demonstrated recurrence rates of advanced adenoma at 3-year follow-up after polypectomy of 5% to 17% for adenomas larger than 1 cm,4
15
22
36 10% to 13% for villous adenomas,4
22
36 10% to 12% for adenomas with high grade dysplasia,4
36 and 9% to 18% for 3 or more adenomas.15
22
36 In the latest U.S. guidelines, patients with advanced adenoma or 3 or more adenomas are recommended to have follow-up surveillance colonoscopy at 3 years.7 In this study, despite the lack of statistical significance, the risk of advanced adenoma recurrence was 2 times higher in patients with baseline advanced adenoma than in patients with baseline non-advanced adenoma, confirming the definitive difference in advanced adenoma recurrence at the 3-year follow-up between patients with baseline advanced adenoma and patients without baseline advanced adenoma. Our study also showed that the presence of 3 or more adenomas at baseline was an independent risk factor for overall adenoma recurrence, confirming that the number of adenomas at baseline colonoscopy should be considered in determining the interval of surveillance colonoscopy. We were unable to find any relationship between other baseline adenoma characteristics such as size, histology, and degree of dysplasia and advanced adenoma recurrence, which may be due to the small number of patients in this study who experienced advanced adenoma recurrence.
Prior reports have shown that an increased risk of advanced adenoma is associated with baseline advanced adenoma.3
15
22
23
24
25
26
27 Nusko et al.27 demonstrated that the recurrence rates of advanced adenoma after polypectomy were 10% at 3 years. In Korean patients with baseline advanced adenoma, Seo et al.37 reported that the 3-year cumulative rates of metachronous advanced neoplasm were 13.2%, while Chung et al.3 reported that the 5-year cumulative rates of advanced adenoma were 12.2%. A pooled analysis that used individual data from 8 North American prospective studies also indicated that patients with baseline advanced adenoma were more likely to develop advanced neoplasm (15.5%) after a median of 4 years of follow-up surveillance.21 Thus, international guidelines such as the U.S. Multi-Society Task Force on Colorectal Cancer,7 American College of Gastroenterology, American Society of Gastrointestinal Endoscopy, and British Society of Gastroenterology38 have recommended follow-up colonoscopy at 3 years after polypectomy in patients with baseline advanced adenoma.5 In the present study, 3-year cumulative recurrence rate of advanced adenoma was low (6.8%) in patients with baseline advanced adenoma; therefore, our results indicate that the current recommendation of 3-year follow-up surveillance colonoscopy after polypectomy in patients with high-risk findings is appropriate.
Our study has several limitations. First, only 29.3% of patients had a surveillance colonoscopic examination during the follow-up period, which may have introduced a possible selection bias. The surveillance group had higher proportions of males, smokers, and alcohol drinkers and was more likely to have purported high CRC risk variables for baseline adenoma, including advanced adenoma, number, size, and villous histology in comparison with the non-surveillance group. We had considered that the surveillance group in our study might have a higher risk potential for advanced adenoma recurrence compared with the baseline enrolled patients. Second, this was not a community-based cohort study because our population was derived from a group of tertiary medical centers. Therefore, a selection bias could not be avoided. Third, we were unable to evaluate other risk factors for colorectal neoplasia after polypectomy, such as obesity, BMI, metabolic syndrome, diet, and physical activity, which may have influenced the recurrence rate of adenomas, and further studies aimed at assessing recurrence in the context of those factors are necessary.
In conclusion, the results of the present Korean prospective multicenter study reveal that a 3-year surveillance interval for patients with baseline advanced adenoma can be considered appropriate. Future studies designed to evaluate the surveillance interval after polypectomy based on both stratified risk factors and cost-effectiveness are warranted in Korea.
Notes
FINANCIAL SUPPORT: This work was supported by The National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0820290).
AUTHOR CONTRIBUTION: D.S.H. conceived and designed the study. C.S.E., J.H.C., and S.H.J. analyzed the data. W.S.C. and C.S.E. wrote the paper. C.S.E., D.I.P., J.S.B., D.H.Y., S.A.J., S.K.L., S.P.H., C.H.P., S.H.L., J.S.J., S.J.S., B.K., and H.S.K. acquisition of data. W.S.C., D.S.H., and C.S.E. review and/or revision of the manuscript.
References
1. Lieberman DA, De Garmo PL, Fleischer DE, Eisen GM, Helfand M. Patterns of endoscopy use in the United States. Gastroenterology. 2000; 118:619–624. PMID: 10702214.

2. Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003; 124:544–560. PMID: 12557158.

3. Chung SJ, Kim YS, Yang SY, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut. 2011; 60:1537–1543. PMID: 21427200.

4. Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007; 133:1077–1085. PMID: 17698067.

5. Kim TO. Optimal colonoscopy surveillance interval after polypectomy. Clin Endosc. 2016; 49:359–363. PMID: 27484812.

6. Bogie R, Sanduleanu S. Optimizing post-polypectomy surveillance: a practical guide for the endoscopist. Dig Endosc. 2016; 28:348–359. PMID: 26179809.

7. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012; 143:844–857. PMID: 22763141.

8. Matsuda T, Chiu HM, Sano Y, Fujii T, Ono A, Saito Y. Surveillance colonoscopy after endoscopic treatment for colorectal neoplasia: From the standpoint of the Asia-Pacific region. Dig Endosc. 2016; 28:342–347. PMID: 26861487.

9. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC;2010.
10. Kim ER, Kim YH. Clinical application of genetics in management of colorectal cancer. Intest Res. 2014; 12:184–193. PMID: 25349592.

11. Cha JM. Colonoscopy quality is the answer for the emerging issue of interval cancer. Intest Res. 2014; 12:110–116. PMID: 25349577.

12. Jang HW, Park SJ, Hong SP, Cheon JH, Kim WH, Kim TI. Risk factors for recurrent high-risk polyps after the removal of high-risk polyps at initial colonoscopy. Yonsei Med J. 2015; 56:1559–1565. PMID: 26446637.

13. Sung JJ, Ng SC, Chan FK, et al. An updated Asia Pacific consensus recommendations on colorectal cancer screening. Gut. 2015; 64:121–132. PMID: 24647008.

14. Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009; 150:1–8. PMID: 19075198.

15. Winawer SJ, Zauber AG, O'Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps: the National Polyp Study Workgroup. N Engl J Med. 1993; 328:901–906. PMID: 8446136.

16. Loeve F, van Ballegooijen M, Snel P, Habbema JD. Colorectal cancer risk after colonoscopic polypectomy: a population-based study and literature search. Eur J Cancer. 2005; 41:416–422. PMID: 15691642.

17. Citarda F, Tomaselli G, Capocaccia R, Barcherini S, Crespi M. Italian Multicentre Study Group. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001; 48:812–815. PMID: 11358901.

18. Alberts DS, Martínez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas: Phoenix Colon Cancer Prevention Physicians' Network. N Engl J Med. 2000; 342:1156–1162. PMID: 10770980.

19. Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003; 348:891–899. PMID: 12621133.
20. Alberts DS, Martínez ME, Hess LM, et al. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J Natl Cancer Inst. 2005; 97:846–853. PMID: 15928305.

21. Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009; 136:832–841. PMID: 19171141.

22. Martínez ME, Sampliner R, Marshall JR, Bhattacharyya AK, Reid ME, Alberts DS. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology. 2001; 120:1077–1083. PMID: 11266371.

23. Huang Y, Gong W, Su B, et al. Recurrence and surveillance of colorectal adenoma after polypectomy in a southern Chinese population. J Gastroenterol. 2010; 45:838–845. PMID: 20336471.

24. Matsuda T, Fujii T, Sano Y, et al. Five-year incidence of advanced neoplasia after initial colonoscopy in Japan: a multicenter retrospective cohort study. Jpn J Clin Oncol. 2009; 39:435–442. PMID: 19483205.

25. Leung WK, Lau JY, Suen BY, et al. Repeat-screening colonoscopy 5 years after normal baseline-screening colonoscopy in average-risk Chinese: a prospective study. Am J Gastroenterol. 2009; 104:2028–2034. PMID: 19455125.

26. Bertario L, Russo A, Sala P, et al. Predictors of metachronous colorectal neoplasms in sporadic adenoma patients. Int J Cancer. 2003; 105:82–87. PMID: 12672034.

27. Nusko G, Mansmann U, Kirchner T, Hahn EG. Risk related surveillance following colorectal polypectomy. Gut. 2002; 51:424–428. PMID: 12171968.

28. Fossi S, Bazzoli F, Ricciardiello L, et al. Incidence and recurrence rates of colorectal adenomas in first-degree asymptomatic relatives of patients with colon cancer. Am J Gastroenterol. 2001; 96:1601–1604. PMID: 11374706.

29. Sass DA, Schoen RE, Weissfeld JL, et al. Relationship of visceral adipose tissue to recurrence of adenomatous polyps. Am J Gastroenterol. 2004; 99:687–693. PMID: 15089903.

30. Jacobs ET, Ahnen DJ, Ashbeck EL, et al. Association between body mass index and colorectal neoplasia at follow-up colonoscopy: a pooling study. Am J Epidemiol. 2009; 169:657–666. PMID: 19147743.

31. Reid ME, Marshall JR, Roe D, et al. Smoking exposure as a risk factor for prevalent and recurrent colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2003; 12:1006–1011. PMID: 14578135.
32. Paskett ED, Reeves KW, Pineau B, et al. The association between cigarette smoking and colorectal polyp recurrence (United States). Cancer Causes Control. 2005; 16:1021–1033. PMID: 16184467.

33. Gao F, Liao C, Liu L, Tan A, Cao Y, Mo Z. The effect of aspirin in the recurrence of colorectal adenomas: a meta-analysis of randomized controlled trials. Colorectal Dis. 2009; 11:893–901. PMID: 19055515.

34. Wei JT, Mott LA, Baron JA, Sandler RS. Polyp Prevention Study Group. Reported use of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors was not associated with reduced recurrence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2005; 14:1026–1027. PMID: 15824186.
35. van Heijningen EM, Lansdorp-Vogelaar I, Kuipers EJ, et al. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology. 2013; 144:1410–1418. PMID: 23499951.
36. Bonithon-Kopp C, Piard F, Fenger C, et al. Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum. 2004; 47:323–333. PMID: 14991494.
37. Seo JY, Chun J, Lee C, et al. Novel risk stratification for recurrence after endoscopic resection of advanced colorectal adenoma. Gastrointest Endosc. 2015; 81:655–664. PMID: 25500328.
38. Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010; 59:666–689. PMID: 20427401.

Table 2
Univariate and Multivariate Analyses on RR of Baseline Demographic Characteristics for Recurrent Advanced Adenoma
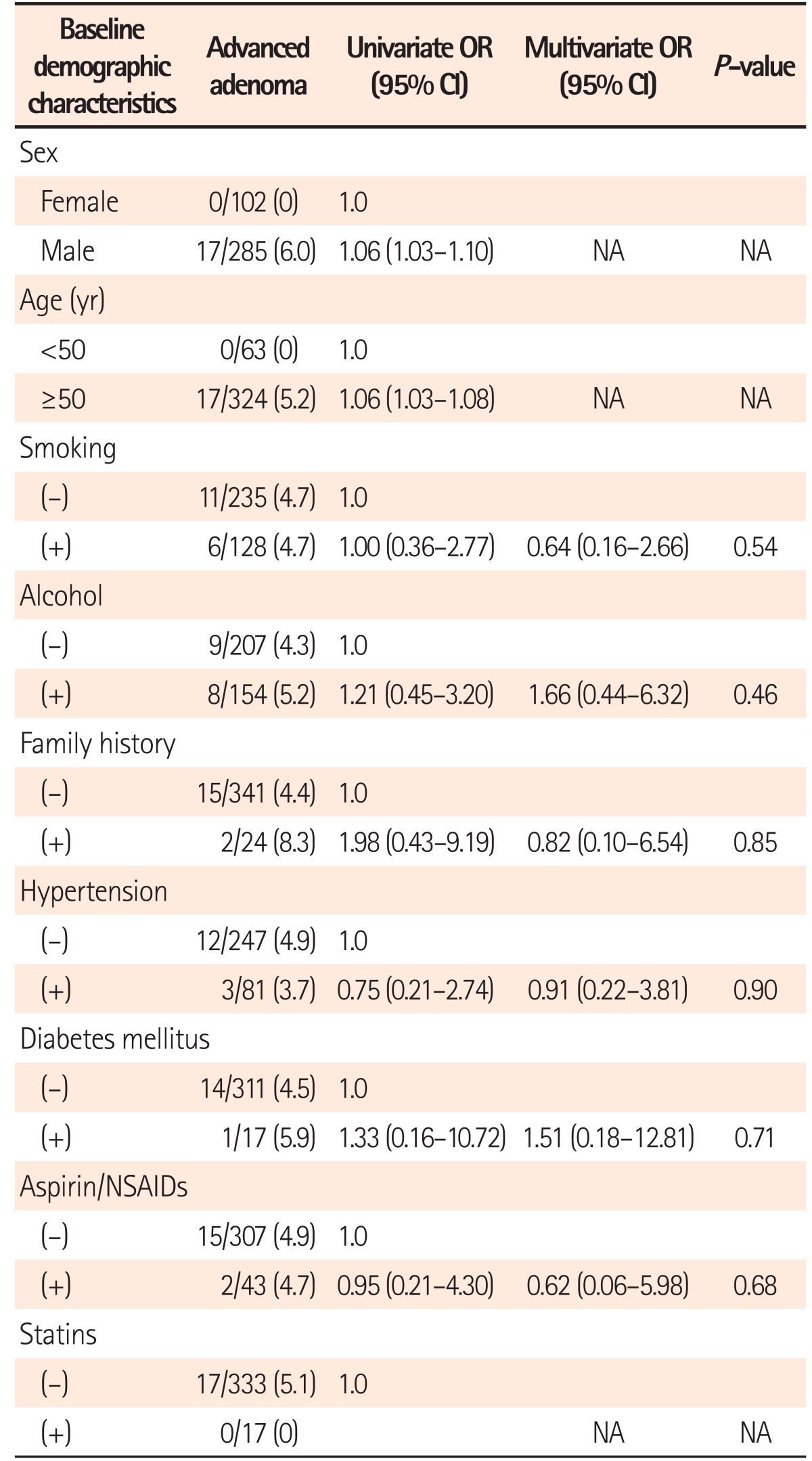
Table 3
Univariate and Multivariate Analyses on RR of Baseline Adenoma Characteristics for Recurrent Advanced Adenoma




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download