1. Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999; 11:1185–1194.
2. Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology. 1992; 102:330–354.

3. Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990; 65:909–911.
4. Madan M, Piplani S, Sharma M, Bhasin TS, Manjari M, Kaur H. Celiac disease: an assessment of subjective variation and diagnostic reproducibility of the various classification systems. Glob J Med Res. 2015; 15(No 1-C):882.
5. Corazza GR, Villanacci V, Zambelli C, et al. Comparison of the interobserver reproducibility with different histologic criteria used in celiac disease. Clin Gastroenterol Hepatol. 2007; 5:838–843.

6. Ensari A. Gluten-sensitive enteropathy (celiac disease): controversies in diagnosis and classification. Arch Pathol Lab Med. 2010; 134:826–836.

7. Cummins AG, Alexander BG, Chung A, et al. Morphometric evaluation of duodenal biopsies in celiac disease. Am J Gastroenterol. 2011; 106:145–150.

8. Meinhard EA, Wadbrook DG, Risdon RA. Computer card morphometry of jejunal biopsies in childhood coeliac disease. J Clin Pathol. 1975; 28:85–93.

9. Taavela J, Koskinen O, Huhtala H, et al. Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PLoS One. 2013; 8:e76163.

10. Kuitunen P, Kosnai I, Savilahti E. Morphometric study of the jejunal mucosa in various childhood enteropathies with special reference to intraepithelial lymphocytes. J Pediatr Gastroenterol Nutr. 1982; 1:525–531.

11. Ciclitira PJ, Evans DJ, Fagg NL, Lennox ES, Dowling RH. Clinical testing of gliadin fractions in coeliac patients. Clin Sci (Lond). 1984; 66:357–364.

12. Tulloh EA, Baylis JM, Challacombe DN. Automated analysis of morphological change in the duodenal mucosa of children with coeliac disease. Arch Dis Child. 1981; 56:860–863.

13. Ghoshal UC, Gwee KA. Post-infectious IBS, tropical sprue and small intestinal bacterial overgrowth: the missing link. Nat Rev Gastroenterol Hepatol. 2017; 14:435–441.

14. Husby S, Koletzko S, Korponay-Szabó IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012; 54:136–160.

15. Adelman DC, Murray J, Wu TT, Mäki M, Green PH, Kelly CP. Measuring change in small intestinal histology in patients with celiac disease. Am J Gastroenterol. 2018; 113:339–347.

16. Elli L, Branchi F, Sidhu R, et al. Small bowel villous atrophy: celiac disease and beyond. Expert Rev Gastroenterol Hepatol. 2017; 11:125–138.

17. Singh P, Kurray L, Agnihotri A, et al. Titers of anti-tissue transglutaminase antibody correlate well with severity of villous abnormalities in celiac disease. J Clin Gastroenterol. 2015; 49:212–217.

18. Rahmati A, Shakeri R, Sohrabi M, et al. Correlation of tissue transglutaminase antibody with duodenal histologic marsh grading. Middle East J Dig Dis. 2014; 6:131–136.
19. Hammer ST, Greenson JK. The clinical significance of duodenal lymphocytosis with normal villus architecture. Arch Pathol Lab Med. 2013; 137:1216–1219.

20. Trejdosiewicz LK. What is the role of human intestinal intraepithelial lymphocytes? Clin Exp Immunol. 1993; 94:395–397.

21. Veress B, Franzén L, Bodin L, Borch K. Duodenal intraepithelial lymphocyte-count revisited. Scand J Gastroenterol. 2004; 39:138–144.

22. Järvinen TT, Collin P, Rasmussen M, et al. Villous tip intraepithelial lymphocytes as markers of early-stage coeliac disease. Scand J Gastroenterol. 2004; 39:428–433.

23. Shalimar DM, Das P, Sreenivas V, Gupta SD, Panda SK, Makharia GK. Mechanism of villous atrophy in celiac disease: role of apoptosis and epithelial regeneration. Arch Pathol Lab Med. 2013; 137:1262–1269.

24. Ghosal UC, Das P. Diagnosis of celiac disease. In : Makharia GK, editor. Handbook of celiac disease. 1st ed. New Delhi: Kontentworx Publications;2015. p. 63–85.
25. Švajdler M, Daum O, Rychlý B. Diagnosing celiac disease: role of the pathologists. Int J Celiac Dis. 2014; 2:70–75.

26. Rosekrans PC, Meijer CJ, Polanco I, Mearin ML, van der Wal AM, Lindeman J. Long-term morphological and immunohistochemical observations on biopsy specimens of small intestine from children with gluten-sensitive enteropathy. J Clin Pathol. 1981; 34:138–144.

27. Boruah D, Bhatia JK, Kamal KD, Malik A. Morphometric changes in jejunal mucosa in HIV positive patients presenting with enteropathy: an Indian study. Ann Pathol Lab Med. 2017; 4:A379–A387.

28. Hegenbart S, Uhl A, Vécsei A. Survey on computer aided decision support for diagnosis of celiac disease. Comput Biol Med. 2015; 65:348–358.

29. Hagendorn E, Whitney-Miller C, Huber A, Potts SJ. Development of a tissue image analysis algorithm for celiac drug development. In : Potts SJ, Eberhard DA, Wharton KA, editors. Methods in pharmacology and toxicology. New York: Springer;2015. p. 141–152.
30. Vécsei A, Amann G, Hegenbart S, Liedlgruber M, Uhl A. Automated Marsh-like classification of celiac disease in children using local texture operators. Comput Biol Med. 2011; 41:313–325.
31. Ciaccio EJ, Tennyson CA, Lewis SK, Krishnareddy S, Bhagat G, Green PH. Distinguishing patients with celiac disease by quantitative analysis of videocapsule endoscopy images. Comput Methods Programs Biomed. 2010; 100:39–48.

32. Gottlieb K, Dawson J, Hussain F, Murray JA. Development of drugs for celiac disease: review of endpoints for Phase 2 and 3 trials. Gastroenterol Rep (Oxf). 2015; 3:91–102.

33. Murray JA, Kelly CP, Green PH, et al. No difference between latiglutenase and placebo in reducing villous atrophy or improving symptoms in patients with symptomatic celiac disease. Gastroenterology. 2017; 152:787–798.
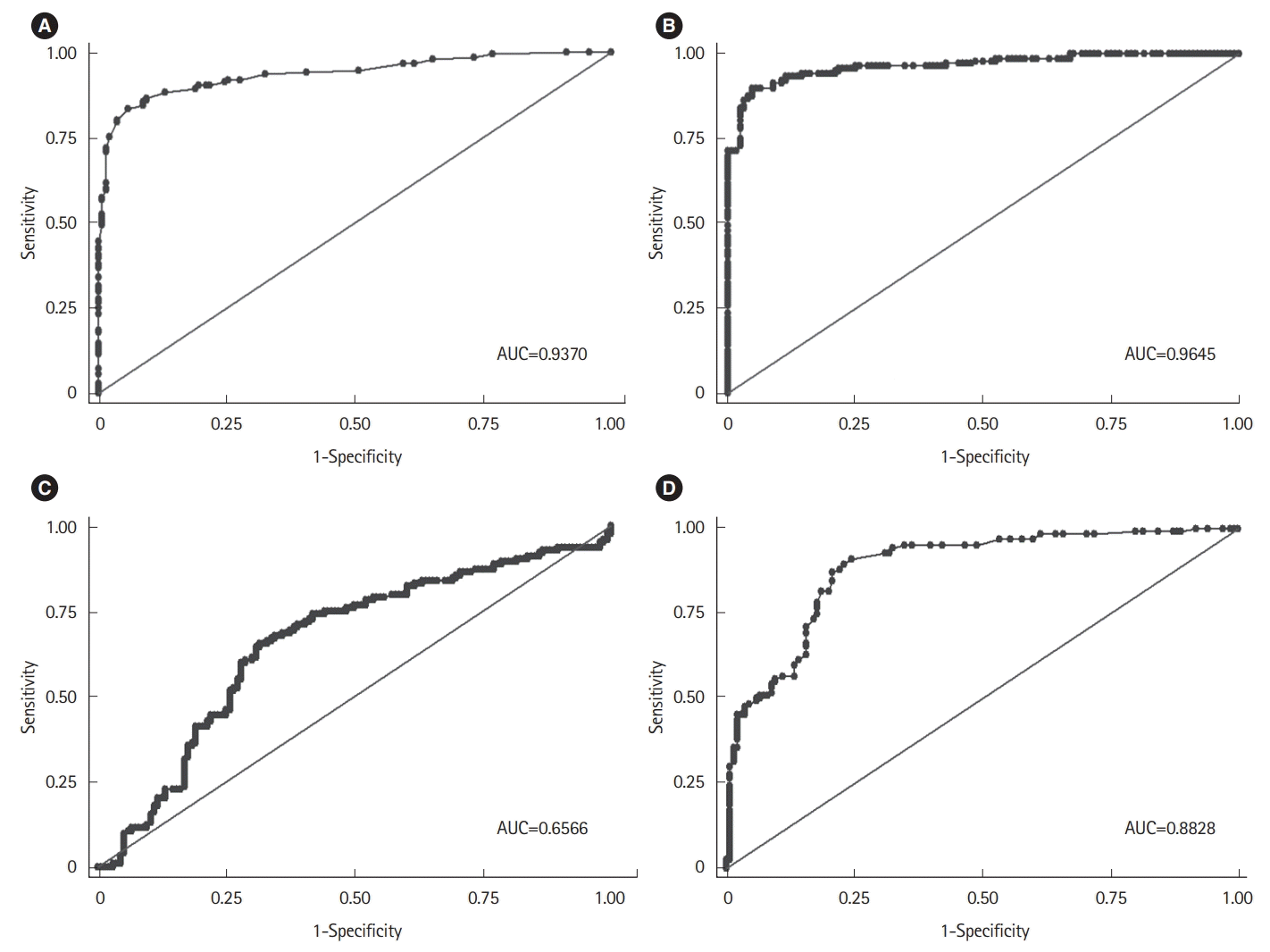
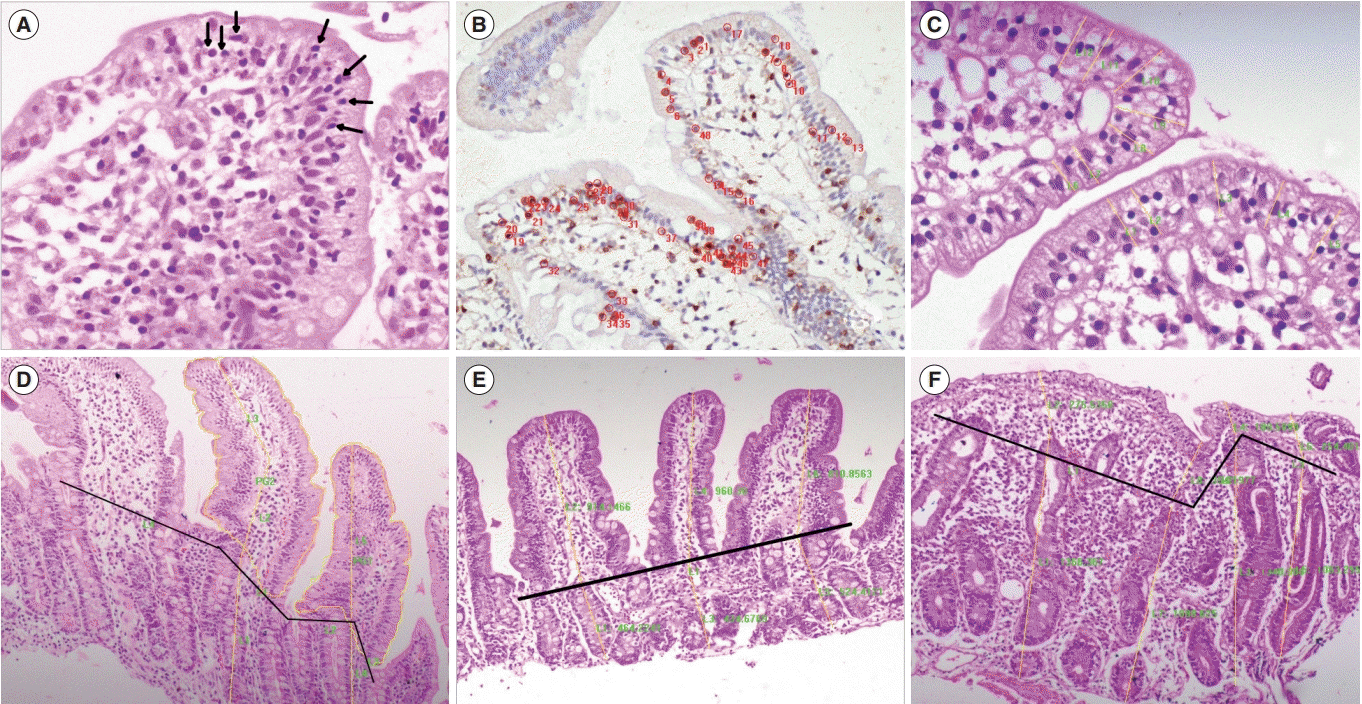




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download