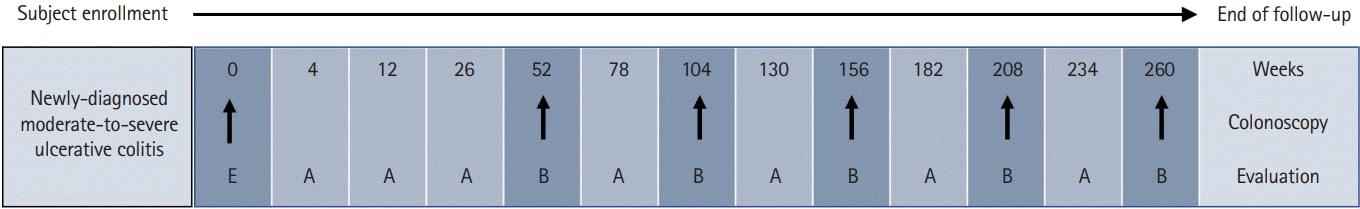
 | Fig. 1.Schematic overview of study follow-up and evaluation. Enrollment visit (E) evaluation items: patient-reported outcomes, assessment by medical staff, laboratory tests, medical resource utilization records, specimen collection and storage; follow-up visit (A): assessment by medical staff, medical resource utilization records; follow-up visit (B): patient-reported outcomes, assessment by medical staff, laboratory tests, medical resource utilization records. |
Ulcerative colitis (UC) is a chronic, disabling IBD with a variable disease course. The incidence of UC is considered to be quite low in Korea, but it has increased substantially in recent years. In a first population-based study conducted in one district of Seoul, the mean annual incidence of UC was reported to be 1.51/100,000 inhabitants (95% CI, 1.34–1.67) over the 20-year study period (1986–2005) [1]. At the end of 2005, the adjusted prevalence of UC was reported as 30.87 per 100,000 inhabitants (95% CI, 27.47–34.27) [1]. Several population-based studies using the Korean National Health Insurance claims database show a significant increase in the incidence and prevalence of UC [2-4]. The mean annual incidence of UC was estimated to be 4.6–5.0/100,000 over 8 years (2006–2014) [2,3]. The prevalence of UC has also increased significantly, ranging from 59.3/100,000 in 2010 to 69.3/100,000 in 2014, and continued increase is anticipated in the future [4].
An escalating burden of UC eventually causes a considerable economic burden for the healthcare system. The first cost-of-illness study for IBD in Korea reported an approximately 2-fold increase in total IBD-related direct healthcare costs over 5 years from $8.5 million in 2010 to $16.9 million in 2014 [4]. In addition to this “double burden” of the disease, UC is also associated with a significant psychosocial burden caused by debilitating symptoms that lead to poor health-related quality of life (HRQL) and loss of work productivity [5]. Finally, patients with UC have a higher risk of developing colorectal cancers, compared with the general population [6].
Given the chronic nature of UC, a comprehensive understanding of the natural history of UC is imperative for improving long-term care. In particular, identifying predictors of poor disease outcomes is essential for preventing disease progression and to efficiently use limited resources by optimizing personalized treatment strategies. In these regards, a prospective cohort study is an ideal research model for identifying the cause, and especially for understanding the natural history, of disease. Large-scale cohort studies in Europe and North America have demonstrated a changing landscape of UC disease course and outcomes. Many useful clinical predictors of severe disease course were also identified in these studies [7].
Further long-term prospective cohort studies for IBD are necessary for better understanding and management of the disease. For example, it is unclear whether evolving therapies or strategies, such as early introduction of biologics and treat-to-target, can truly alter disease course and improve the long-term disease-related outcomes. Similarly, it must be verified whether the natural history of IBD revealed in Western studies can be applied to Asian patients, given the different genetic backgrounds or environments among populations [8]. It is reported that Asian patients with UC may have a more favorable prognosis than Westerners [6]. A recent meta-analysis of population-based cohort studies showed significant variations among phenotypes and outcomes of IBD across ethnic groups, reinforcing the need for further, long-term prospective cohort studies in non-Caucasian patients [9].
The moderate-to-severe ulcerative colitis in Korea (MOSAIK) cohort study is the first nationwide prospective cohort study on UC in Korea. The study is designed to capture all eligible patients with newly-diagnosed UC in referred to tertiary hospitals (hospital inception cohort study), and is generally aimed at exploring individual disease course and identifying treatment response and prognostic factors. The MOSAIK cohort study prospectively collects all relevant standardized clinical data at both baseline and regularly scheduled 5-year followup visits. Disease activity and extent are evaluated by validated indices, such as Mayo score and Montreal classification [10].
The MOSAIK study also collects extensive patient-reported outcome (PRO) data via patient surveys. PRO data items include the following: (1) a hospital anxiety and depression scale to assess emotional health; (2) a work productivity and activity impairment questionnaire to assess work disability; and (3) an IBD Questionnaire and 12-item short-form health survey to assess disease-specific and generic HRQL. The patient survey also collects information on dietary patterns and complementary and alternative medical treatment. All data are transferred via the web to a central database (https://www.cubecdms.com). Fig. 1 depicts the schematic overview of the study follow-up and evaluation in the MOSAIK cohort study.
Blood samples are collected from patients who have consented for genetic or biomarker investigations. Samples are transferred to a central laboratory, where genomic DNA and sera are separately stored for up to 10 years. These bio-samples will serve as the basis for a variety of collaborating translational research that will greatly enhance identification of both pathogenic mechanisms and individual predictors of disease course.
The initial meeting for the MOSAIK cohort study was held on July 12, 2013. Thereafter, a working group of 12 faculty gastroenterologists from 10 academic hospitals, who are all members of the Korean Association for the Study of Intestinal Diseases (KASID), developed the study protocol. The study protocol includes a total of 834 standardized data items and their definitions.
A constructive discussion on study inclusion criteria focused on the question of “Why not mild colitis?” As stated above, the study included only moderate-to-severe colitis. Tertiary-level, academic hospitals are the facilities where patients with moderate-to-severe disease are usually referred after visiting facilities with lower levels of care, such as primary clinics or secondary hospitals. The original study protocol was approved by all investigators at the study initiation meeting on March 15, 2014, and it was registered at www.clinicaltrials.gov (Clinical-Trials.gov identifier: NCT02229344). Since the initial meeting, the MOSAIK cohort study has been led by professor from Kyung Hee University (H.J.K.), Korea, who was president of the KASID at the beginning of the study.
The first patient consented to participate in the study on 25 August, 2014, as of March 2017, study enrolled 355 newly-diagnosed patients with moderate-to-severe UC. As of December 2018, 174 investigators (32 principal investigators, 65 subinvestigators, 70 study coordinators, 2 nurses, 6 lab technicians) from 30 tertiary hospitals in Korea have participated in this study. The enrollment of eligible patient is now complete, and follow-up investigations are ongoing. The last visit of the last study subject is expected to be on February 28, 2022.
Baseline demographics and clinical characteristics of the MOSAIK cohort were completely analyzed last year and results presented at the 6th annual meeting of the Asian Organization for Crohn’s and Colitis (AOCC), January 21 to 23, 2018, Shanghai, China. Additional results concerning baseline PRO data from the MOSAIK cohort was presented at the annual congress of the KASID, April 13 to 14, 2018, Seoul, Korea. The analysis of 1-year follow-up results will be completed soon, and the final report and publication of major findings are expected circa February 2023. Table 1 summarizes milestones and schedules for completion of the study.
Milestones and Future Schedules of the MOSAIK Cohort Study in Korea
In summary, the MOSAIK cohort study is a well-designed, large, prospective and observational study that collects unique data to assess the natural history of newly-diagnosed, moderate-to-severe UC in Korea. The study has collected extensive standardized clinical data to identify useful predictors of poor disease outcomes. Uniquely, the study prospectively collected PRO data using a regular comprehensive patient survey. All the data collected are under strict quality control and cleaning, which ensures data consistency. The MOSAIK study will provide high-quality data to meet the needs of different research fields and topics.
Till date, the MOSAIK cohort study has collected 201 matched bio-samples with accurate clinical metadata. The first study using these bio-samples is underway in collaboration with the Korea Center for Disease Control and Prevention. The study will perform genome-wide association analysis using the single nucleotide polymorphism (SNP) microarray platform with preexisting SNP microarray data of population cohorts as the control group. Integrative analysis of genome-wide association study (GWAS) hotspots and functional genomics data will explore the possible trajectory from genotype to phenotype in Korean patients with UC.
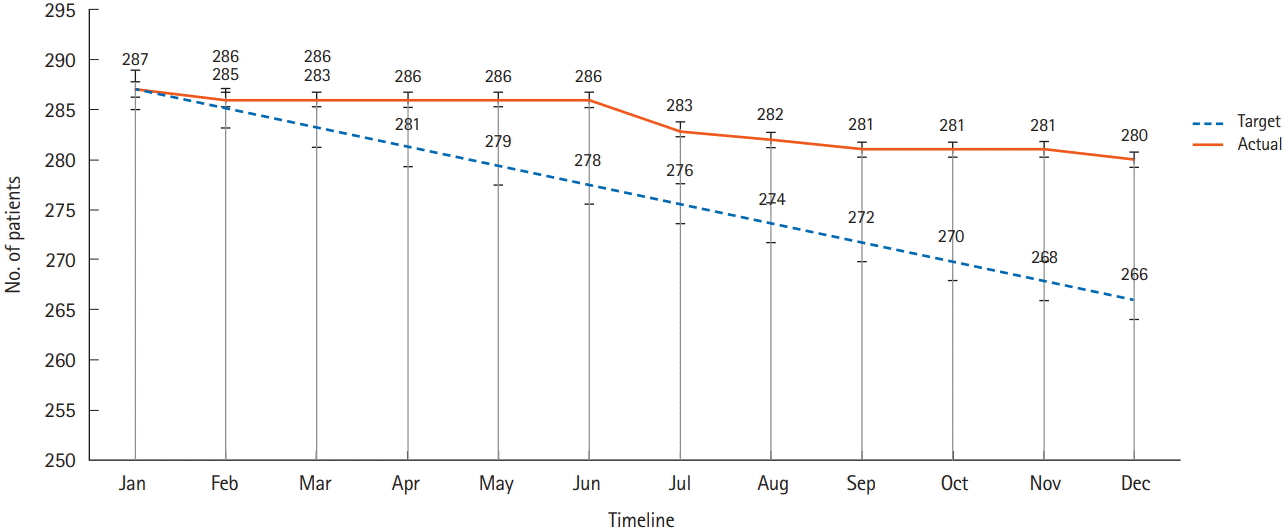
The MOSAIK cohort study has some limitations. First, loss during follow-up is the biggest problem of any prospective cohort study, and this study is no exception. As of December 2018, a total of 280 patients have been actively followed (Fig. 2). Second, the study has not collected stool or colonic tissue for gut microbiome analyses due to limited resources. Further efforts are needed to secure samples that can support a variety of constantly changing research topics and analytical platforms. Securing new research funding will be necessary to accomplish these goals.
Ultimately, the MOSAIK cohort study has opened new opportunities for innovative IBD research in Korea. All researchers who have actively participated in study may submit research proposals annually. We anticipate that the MOSAIK cohort study will serve as a cornerstone for nationwide research on UC and also become a landmark IBD cohort study in the future.
Notes
FINANCIAL SUPPORT
The moderate-to-severe ulcerative colitis in Korea (MOSAIK) cohort study has been funded and supported by Janssen Korea.
AUTHOR CONTRIBUTION
Lee CK: conception and design of the study, composing the first draft of the paper. Lee KM, Park DI, Jung SA, Jeen YT, Park YS: conception and design of the study, critical revision of the manuscript for important intellectual content. Kim HJ: conception and design of the study, critical revision of the manuscript for important intellectual content and final approval of the version to be submitted.
Go to : 
ACKNOWLEDGMENTS
The authors acknowledge and would like to thank the following members of the MOSAIK study group for participating in the study: Sung Noh Hong, Sungkyunkwan University School of Medicine, Seoul; Jong Pil Im, Seoul National University College of Medicine, Seoul; Byong Duk Ye, University of Ulsan College of Medicine, Ulsan; Jae Myung Cha, Kyung Hee University College of Medicine, Seoul; Jae Hee Cheon, Yonsei University College of Medicine, Seoul; Yoon Jae Kim, Gachon University Gil Medical Center, Incheon; Tae Oh Kim, Inje University College of Medicine, Busan; Geom Seog Seo, Wonkwang University School of Medicine, Iksan; Ja Seol Koo, Korea University College of Medicine, Seoul; Joo Sung Kim, Seoul National University College of Medicine, Seoul; Byung Ik Jang, Yeungnam University College of Medicine, Daegu; Jeong Eun Shin, Dankook University College of Medicine, Cheonan; Ji Won Kim, Seoul National University Boramae Hospital, Seoul; Young Su Park, Seoul National University Bundang Hospital, Seongnam; Heejung Lee, Medical Affairs, Janssen Korea, Seoul, Korea.
Go to : 
REFERENCES
1. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis. 2008; 14:542–549.

2. Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015; 21:623–630.

3. Jung YS, Han M, Kim WH, Park S, Cheon JH. Incidence and clinical outcomes of inflammatory bowel disease in South Korea, 2011-2014: a nationwide population-based study. Dig Dis Sci. 2017; 62:2102–2112.

4. Kim JW, Lee CK, Rhee SY, Oh CH, Shim JJ, Kim HJ. Trends in health-care costs and utilization for inflammatory bowel disease from 2010 to 2014 in Korea: a nationwide populationbased study. J Gastroenterol Hepatol. 2018; 33:847–854.

5. Hoivik ML, Moum B, Solberg IC, et al. Health-related quality of life in patients with ulcerative colitis after a 10-year disease course: results from the IBSEN study. Inflamm Bowel Dis. 2012; 18:1540–1549.

6. Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V. Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017; 2:269–276.

7. Wanderås MH, Moum BA, Høivik ML, Hovde Ø. Predictive factors for a severe clinical course in ulcerative colitis: results from population-based studies. World J Gastrointest Pharmacol Ther. 2016; 7:235–241.

8. Lee JW, Im JP, Cheon JH, Kim YS, Kim JS, Han DS. Inflammatory bowel disease cohort studies in Korea: present and future. Intest Res. 2015; 13:213–218.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download