Abstract
The incidence of pediatric inflammatory bowel disease (IBD) is increasing worldwide, especially in the developing countries. It differs from adult disease in clinical manifestations, especially with regard to genetic predisposition in monogenic IBD. Pediatric disease also have a tendency to show more aggressive inflammation and greater extent of lesion. Newer drugs such as antitumor necrosis factor-α have been known to make a difference in treating pediatric IBD. Recent studies suggested that the patients with high risk factors might have some benefits from earlier use of biologics. To achieve treatment goals such as relieving symptoms, optimizing growth, and improving quality of life while minimizing drug toxicity, more research is needed to develop tools for risk stratification in the use of biologics for pediatric IBD.
Pediatric IBD is a growing concern in pediatric health care. Nearly a quarter of all patients with IBD develop the disease during childhood [1]. In recent decades, the incidence and prevalence of pediatric IBD have increased and the highest incidence has been reported from Canada, Norway, Sweden, Finland, the United Kingdom, and Ireland [2]. The incidence and prevalence of pediatric IBD in Singapore showed a 10-fold rise from 0.23 to 2.28 per 100,000 in the past 20 years, even though previously published data on the incidence of IBD in Asia showed differences among countries [3,4]. In Korea, recently published local data showed a rapidly rising trend in the incidence between 2011 and 2016; the incidence for all pediatric IBD increased from 0.86 to 3.33 per 100,000, with an increase from 0.67 to 2.78 for CD and from 0.19 to 0.56 for UC [5]. After the introduction of anti-TNF-α blockers for use in IBD, pediatric IBD showed dramatically improved outcomes, similar to those in adults. However, it is still difficult for physicians to understand the current strategy for treatment of pediatric IBD, because of the lack of information and experience. In this article, clinical aspects and treatment of pediatric IBD will be discussed in the context of biologics.
Childhood-onset IBD seems to be a more aggressive and rapidly progressive disease compared to adult-onset IBD [6,7]. CD is more prevalent than UC in children. The ratio of boys to girls is as high as 1.8:1. The most common type of disease distribution is pan-enteric or pan-colic. These cases were more often treated with systemic steroids and azathioprine and had a higher frequency of steroid dependence. The patients showed a more severe disease course compared to that in adults with IBD. These patients were more likely to have upper GI involvement, extraintestinal manifestations, and stricturing and penetrating disease. Among pediatric IBD patients, 44% required surgery at some point, with a 34% risk within the first 5 years after diagnosis [7-10]. In CD, no differences were found when comparing corticosteroid responsiveness between pediatric and adult patients; however, the inflammatory phenotype is more common than the stricturing or penetrating phenotype in childhood [11,12].
Among patients with IBD onset at a young age, 29% have 1 or more family members with IBD. The subgroup of children younger than 3 years of age with UC had the highest prevalence of first-degree relatives with IBD (44%) [13]. Several genetic defects that disturb intestinal epithelial barrier function or affect innate and adaptive immune function have incomplete penetrance of the IBD-like phenotype [14]. Monogenic defects, especially those affecting the interleukin-10 (IL-10) signaling pathway, result in severe or intractable disease [15]. Patients with IL-10 pathway defects often show initial presentation before 1 year of age. Intractable perianal fistula is a cardinal manifestation and diarrhea with bloody stools is also common with this defect [16,17]. There is no specific treatment for IL-10 pathway defects, except for hematopoietic stem cell transplantation [18,19].
Very-early-onset IBD (VEOIBD) is usually defined when IBD occurs in children less than 6 years of age. A child less than 2 years of age can be classified as having infantile IBD [20]. However, in the Paris classification, which is a modified pediatric version of the Montreal classification and is frequently cited in textbooks, pediatric onset IBD is only classified as A1a and A1b, which occur at less than 10 years of age or between age 10 and 17 years, respectively (Table 1) [21]. Recent advances in translational research and next generation sequencing or whole exome sequencing have made it possible to change the diagnosis and treatment in VEOIBD. A gene panel or gene chip showed promising results in the diagnosis of VEOIBD [14]. The clinical course of monogenic IBD, which is a subgroup of VEOIBD, is more severe than adolescent onset disease. The initial Pediatric Crohn’s Disease Activity Index (PCDAI) and Pediatric Ulcerative Colitis Activity Index (PUCAI) scores, the annual incidence of surgery, and the number of hospitalization per year were higher in the monogenic IBD group than that in other IBD groups [22]. There is no specific treatment for VEOIBD; however, a few reports showed that hematopoietic stem cell transplantation showed effectiveness in IL-10 receptor deficiency and XIAP mutations. VEOIBD could be useful in identifying the pathophysiology of IBD, because pediatric cases have a relatively stronger genetic background and less exposure to environmental and behavioral influences than adults [23,24]. As recent advances in VEOIBD make this category important in pediatric IBD, the Paris classification alone is unable to categorize all patient groups.
The traditional treatment strategy based on clinical symptom improvement does not improve long-term outcomes in CD and patients cannot avoid bowel damage. Therefore, the “treat to target” concept was introduced to incorporate use of biological markers and mucosal healing into IBD treatment [25]. This new method was adopted from the experience with rheumatic diseases, and can be tentatively regarded as an active approach to the severe disease group [26]. However, there are insufficient data with regard to optimal indications, biomarkers, and treatment strategies, especially in children. Early introduction of biologics in patients with poor prognostic factors, such as deep colonic ulcerations, extensive disease, marked growth retardation, severe osteoporosis, B2 and/or B3 behavior, and severe perianal disease, can be recommended to reduce bowel damage [27].
Endoscopic evaluation is the gold standard for diagnosis of CD in children. However, esophagogastroduodenoscopy and colonoscopy are more difficult and dangerous in children. To overcome the gap between the need to frequently evaluate disease severity and the difficulty of performing endoscopy in children, the PCDAI and PUCAI have been developed [28,29]. The PCDAI includes growth in children as an important parameter. These scoring systems are often used to determine treatment parameters in many clinical settings and investigational trials, as well as for insurance reimbursement (Tables 2 and 3).
The aims of therapy in pediatric IBD traditionally have been to relieve symptoms, optimize growth, and improve quality of life while minimizing drug toxicity [27]. To ensure growth in pediatric patients with CD, aggressive control of inflammation is essential. A recent consensus about the achievement of mucosal healing, especially in patients with poor prognostic factors, could not be fully supported because of the lack of evidence. It is difficult to identify patients with definite risk in order to initiate early aggressive immunotherapy and to define the necessary degree of mucosal healing and depth of transmural healing [27]. Current recommended strategy in pediatric CD is based on escalating medical therapy, beginning with nutritional intervention and/or steroids to achieve targets [30]. Overall, no differences in drug response were found when comparing pediatric and adult CD patients; therefore, current treatment options based on steroid responsiveness can be the same in adults and children. However, it is very important to avoid steroids in children as much as possible (Fig. 1).
Nutritional intervention in pediatric patients is essential to control the disease, especially in CD. Exclusive enteral nutrition is recommended as first-line therapy to induce remission in children with active luminal CD. However, there is no evidence for the use of nutritional intervention in fistulizing CD or pediatric UC. The possibility of the development of colon cancer in pediatric IBD should be kept in mind. Even though pediatric colon cancer is very rare, children with VEOIBD can develop colon cancer at an earlier age than expected. In our institution, we reported a patient with VEOIBD and sigmoid colon cancer a few years ago [31].
The transition from pediatric to adult clinical care in IBD has been problematic. Transition requires careful coordination and collaboration among key persons in a multidisciplinary team, including the patient as well as the parents/caregivers and providers. Adult gastroenterologists who participate in the care of young adults should develop competence in key areas of adolescent and young adult care and should make an effort to collaborate with the pediatrician. Providing adequate care for transitioning patients includes education for the development of self-management skills and developmental processes relevant to young adults with IBD [32]. Recent models suggested by European groups should be reviewed by Korean academic societies, and further prospective research is needed [33,34].
The incidence of pediatric IBD is increasing worldwide, especially in the developing countries. It differs from adult disease in clinical manifestations, especially with regard to genetic predisposition in monogenic IBD. To achieve treatment goals of relieving symptoms, optimizing growth, and improving quality of life while minimizing drug toxicity, more research is needed to develop tools for risk stratification in pediatric IBD.
Notes
REFERENCES
1. Kelsen J, Baldassano RN. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis. 2008; 14 Suppl 2:S9–S11.

2. Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011; 17:423–439.

3. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013; 145:158–165. e2.

4. Ong C, Aw MM, Liwanag MJ, Quak SH, Phua KB. Rapid rise in the incidence and clinical characteristics of pediatric inflammatory bowel disease in a South-East Asian cohort in Singapore, 1994-2015. J Dig Dis. 2018; 19:395–403.

5. Hong SJ, Cho SM, Choe BH, et al. Characteristics and incidence trends for pediatric inflammatory bowel disease in Daegu-Kyungpook Province in Korea: a multi-center study. J Korean Med Sci. 2018; 33:e132.

6. Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008; 135:1114–1122.

7. Sauer CG, Kugathasan S. Pediatric inflammatory bowel disease: highlighting pediatric differences in IBD. Gastroenterol Clin North Am. 2009; 38:611–628.

8. Rinawi F, Assa A, Hartman C, et al. Incidence of bowel surgery and associated risk factors in pediatric-onset Crohn’s disease. Inflamm Bowel Dis. 2016; 22:2917–2923.
9. Ruemmele FM, Turner D. Differences in the management of pediatric and adult onset ulcerative colitis: lessons from the joint ECCO and ESPGHAN consensus guidelines for the management of pediatric ulcerative colitis. J Crohns Colitis. 2014; 8:1–4.

10. Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 2014; 63:423–432.
11. Jakobsen C, Bartek J Jr, Wewer V, et al. Differences in phenotype and disease course in adult and paediatric inflammatory bowel disease: a population-based study. Aliment Pharmacol Ther. 2011; 34:1217–1224.

12. Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology. 2008; 135:1106–1113.
13. Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005; 146:35–40.

14. Uhlig HH, Schwerd T, Koletzko S, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014; 147:990–1007. e3.
15. Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009; 361:2033–2045.
16. Shim JO, Hwang S, Yang HR, et al. Interleukin-10 receptor mutations in children with neonatal-onset Crohn’s disease and intractable ulcerating enterocolitis. Eur J Gastroenterol Hepatol. 2013; 25:1235–1240.

17. Shim JO, Seo JK. Very early-onset inflammatory bowel disease (IBD) in infancy is a different disease entity from adultonset IBD; one form of interleukin-10 receptor mutations. J Hum Genet. 2014; 59:337–341.

18. Engelhardt KR, Shah N, Faizura-Yeop I, et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2013; 131:825–830.

19. Ko JS. Is infantile inflammatory bowel disease curable with hematopoietic stem cell transplantation? Korean J Gastroenterol. 2013; 62:313–314.

20. Snapper SB. Very-early-onset inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2015; 11:554–556.
21. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011; 17:1314–1321.

22. Kim KY, Lee EJ, Kim JW, et al. Higher morbidity of monogenic inflammatory bowel disease compared to the adolescent onset inflammatory bowel disease. Pediatr Gastroenterol Hepatol Nutr. 2018; 21:34–42.

23. Tsianos EV, Katsanos KH, Tsianos VE. Role of genetics in the diagnosis and prognosis of Crohn’s disease. World J Gastroenterol. 2012; 18:105–118.

24. Bianco AM, Girardelli M, Tommasini A. Genetics of inflammatory bowel disease from multifactorial to monogenic forms. World J Gastroenterol. 2015; 21:12296–12310.

25. Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol. 2015; 13:1042–1050. e2.

26. Kang B, Choe YH. Early biologic treatment in pediatric Crohn’s disease: catching the therapeutic window of opportunity in early disease by treat-to-target. Pediatr Gastroenterol Hepatol Nutr. 2018; 21:1–11.

27. Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014; 8:1179–1207.
28. Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity Index. J Pediatr Gastroenterol Nutr. 1991; 12:439–447.

29. Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a Pediatric Ulcerative Colitis Activity Index: a prospective multicenter study. Gastroenterolog. 2007; 133:423–432.

30. Danese S, Colombel JF, Reinisch W, Rutgeerts PJ. Review article: infliximab for Crohn’s disease treatment: shifting therapeutic strategies after 10 years of clinical experience. Aliment Pharmacol Ther. 2011; 33:857–869.

31. Noh SY, Oh SY, Kim SH, Kim HY, Jung SE, Park KW. Fifteenyear-old colon cancer patient with a 10-year history of ulcerative colitis. World J Gastroenterol. 2013; 19:2437–2440.

32. Philpott JR, Kurowski JA. Challenges in transitional care in inflammatory bowel disease: a review of the current literature in transition readiness and outcomes. Inflamm Bowel Dis. 2019; 25:45–55.

Fig. 1.
Simplified treatment algorithm for pediatric CD according to risk factors. Dashed line indicates early use of biologics, that is, the “top down” strategy. High-risk factors in children for luminal CD are deep colonic ulcerations, extensive disease, marked growth retardation, severe osteoporosis, B2 and/or B3 behavior, and severe perianal disease [27]. Generally accepted risk factors are a history of more than 2 steroid courses, steroid dependence, hospitalization, chronic (>12 months) symptoms, need for immunosuppressants or need for surgery, terminal ileal location, stricturing and penetrating behavior, smoking, positive serologic markers such as anti-Saccharomyces cerevisiae antibody/perinuclear antineutrophil cytoplasmic antibodies, positive genetic markers such as NOD2/IBD5, and elevated CRP.30 TNF, tumor necrosis factor; ASA, aminosalicylic acid.
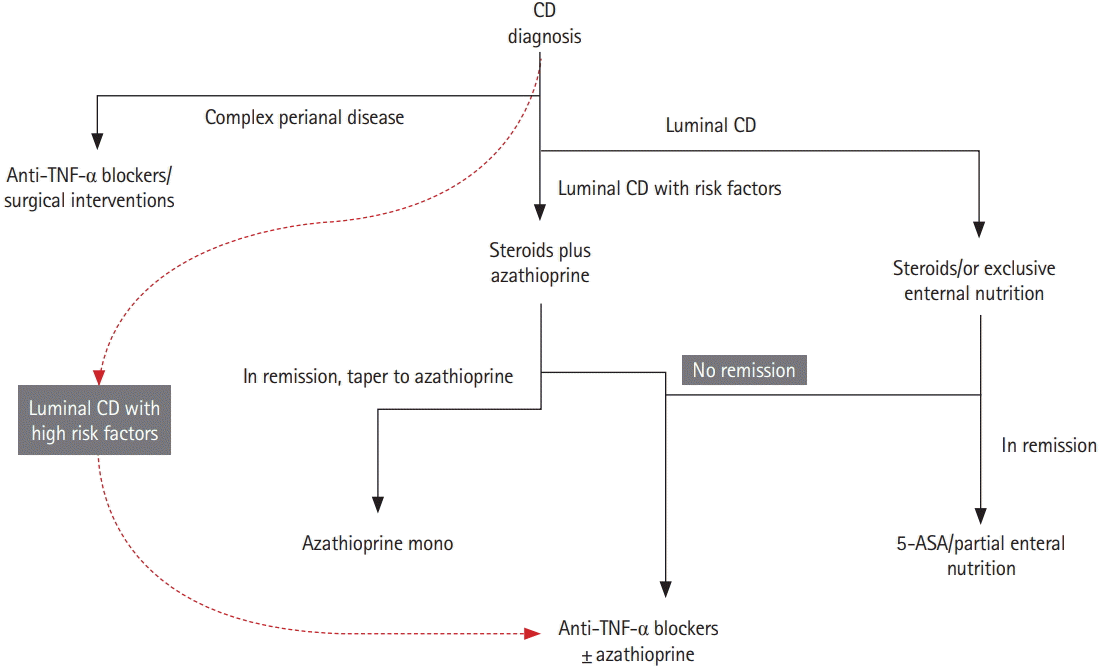
Table 1.
Montreal and Paris Classification of CD
Adapted from Levin A et al. Inflamm Bowel Dis 2011;17:1314-1321. [21]
Table 2.
Pediatric Crohn’s Disease Activity Index
Table 3.
Pediatric Ulcerative Colitis Activity Index




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download