Abstract
Background/Aims
Methods
Results
Notes
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
AUTHOR CONTRIBUTION
All authors have contributed to and agreed on the content of the manuscript, and the respective roles of each author are listed below:
Sood A and Mahajan R: conception and design; collection, analysis and interpretation of the data; drafting of the article; critical revision of the article for important intellectual content; final approval of the article. Juyal G: analysis and interpretation of the data; drafting of the article; critical revision of the article for important intellectual content; final approval of the article. Midha V: conception and design; analysis and interpretation of the data; drafting of the article; critical revision of the article for important intellectual content; final approval of the article. Grewal CS: collection, analysis and interpretation of the data, critical revision of the article for important intellectual content; final approval of the article. Mehta V: analysis and interpretation of the data, critical revision of the article for important intellectual content; final approval of the article. Singh A: collection, analysis and interpretation of the data; drafting of the article; critical revision of the article for important intellectual content; final approval of the article. Joshi MC: drafting of the article; critical revision of the article for important intellectual content; final approval of the article. Narang V, Kaur K, and Sidhu H: data analysis, critical revision of the article for important intellectual content; final approval of the article.
REFERENCES
Fig. 2.
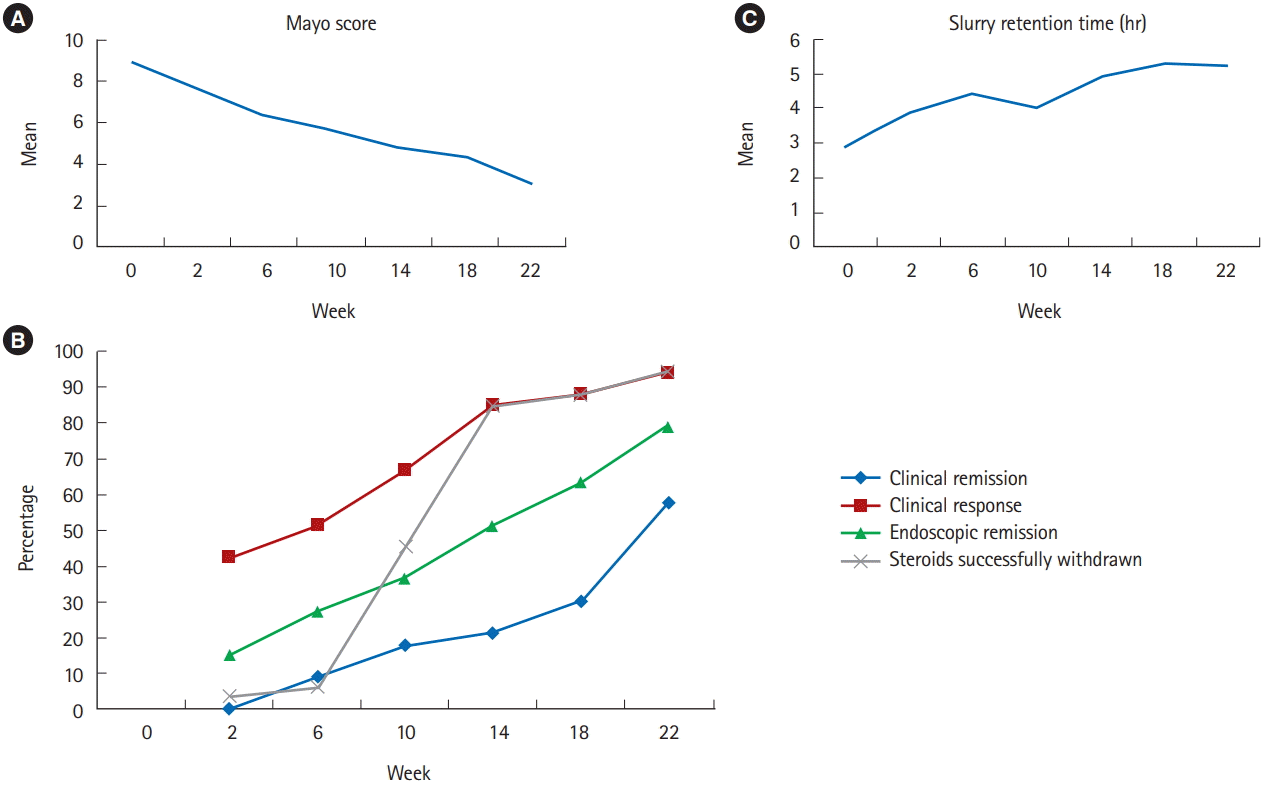
Table 1.
| Characteristic | Case (n=41) |
|---|---|
| Age (yr) | 36.5±10.7 |
| Male sex | 24 (58.5) |
| Disease duration (yr) | 4.6±4.2 |
| Mayo score | 8.8±2.6 |
| Disease extent | |
| E1 | 7 (17.1) |
| E2 | 17 (41.5) |
| E3 | 17 (41.5) |
| Disease severity | |
| Mild | 4 (9.8) |
| Moderate | 37 (90.3) |
| Concomitant medication | |
| Mesalamine | 41 (100) |
| Corticosteroids | 41 (100) |
| Immunosuppressants (AZA) | 22 (53.7) |
| Previous exposure to biological | 12 (29.3)a |
Table 2.
| Timing of FMT | Week 0 | Week 2 | Week 6 | Week 10 | Week 14 | Week 18 | Week 22 |
|---|---|---|---|---|---|---|---|
| Mayo score | 8.9±2.5 | 7.7±1.9 | 6.4±2.3 | 5.7±1.9 | 4.8±1.9 | 4.4±1.9 | 3.1±1.7 |
| Clinical remission | - | 0 | 3 (9.1) | 6 (18.2) | 7 (21.2) | 10 (30.3) | 19 (57.6) |
| Clinical response | - | 11 (42.3) | 17 (51.5) | 22 (66.7) | 28 (84.8) | 29 (87.9) | 31 (93.9) |
| Endoscopic remissiona | - | 4 (15.3) | 9 (27.3) | 12 (36.4) | 17 (51.5) | 21 (63.4) | 26 (78.8) |
| Steroids successfully withdrawn | - | 1 (3.9) | 2 (6.1) | 15 (45.5) | 28 (84.8) | 29 (87.9) | 31 (93.9) |
| Slurry retention time (hr) | 2.9±1.7 | 3.9±2.3 | 4.4±3.6 | 4.0±1.6 | 4.9±3.3 | 5.3±3.1 | 5.2±2.8 |
Table 3.
| Study | Type of study | Country | No. of patients | Disease status | Donor | Route of administration | Frequency | Time of assessment (wk) | Clinical remission (%) | Clinical response (%) | Endoscopic remission (%) | Serious adverse events (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moayyedi et al. [16] | RCT | Canada | 38 | Mild-moderate UC | Spouse (1 patient), volunteers (6 for other patients) | Rectal enema | Weekly-6 wk | 7 | 24.0 | 39.0 | 23.7 | 13.0 |
| Rossen et al. [17] | RCT | The Netherlands | 23 | Mild-moderate UC | Healthy partners, relatives or volunteers | Nasoduodenal tube | Twice (0, 3 wk) | 12 | 30.4 | 47.8 | 8.7 | 8.7 (unrelated to FMT) |
| Costello et al. [18] | RCT | Australia | 38 | Mild-moderate UC | Pooled donor stool (3–4 donors) | Colonoscopy followed by enemas | Baseline colonoscopy (wk 0), then 2 enemas at day 7 | 8 | 50.0 | - | 55.3 | 7.9 |
| Paramsothy et al. [19] | RCT | Australia | 41 | Mild-moderate UC | Pooled donor stool (3–7 donors) | Colonoscopy followed by enemas | Baseline colonoscopy (wk 0), then 5 enemas weekly for 8 wk | 8 | 43.9 | 54.0 | 12.0 | 4.9 |
| Present study | Real life cohort | India | 41 | Steroid dependent | Unrelated healthy volunteers | Colonoscopy | First 2 sessions fortnightly and then every 4 wk till 22 wk | 20 | 41.2 | 76.5 | 61.8 | None |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download