This article has been
cited by other articles in ScienceCentral.
Abstract
Background/Aims
Oral mesalazine is an important treatment for ulcerative colitis (UC), and non-adherence to mesalazine increases the risk of relapse. Controlled-release (CR) mesalazine has 2 formulations: tablets and granules. The relative acceptabilities of these formulations may influence patient adherence; however, they have not been compared to date. This study aimed to evaluate the acceptabilities of the 2 formulations of CR mesalazine in relation to patient adherence using a crossover questionnaire survey.
Methods
UC patients were randomly assigned to 2 groups in a 1:1 ratio. Patients in each group took either 4 g of CR mesalazine tablets or granules for 6 to 9 weeks, and then switched to 4 g of the other formulation for a further 6 to 9 weeks. The acceptability and efficacy were evaluated by questionnaires, and adherence was assessed using a visual analog scale. The difference in acceptabilities between the 2 formulations and its impact on adherence were assessed.
Results
A total of 49 patients were prospectively enrolled and 33 patients were included in the analysis. Significantly more patients found the tablets to be less acceptable than the granules (76% vs. 33%, P=0.0005). The granules were preferable to the tablets when the 2 formulations were compared directly (73% vs. 21%, P=0.004), for their portability, size, and numbers of pills. The adherence rate was slightly better among patients taking the granules (94% vs. 91%) during the observation period, but the difference was not significant (P=0.139).
Conclusions
CR mesalazine granules are more acceptable than tablets, and may therefore be a better option for long-term medication.
Keywords: Colitis, ulcerative, Mesalamine, Medication adherence, Patient acceptance of health care, Drug compounding
INTRODUCTION
Ulcerative colitis (UC) is a life-long disorder of the colon, characterized by a relapsing–remitting course [
1]. The optimal goal of medical treatment in UC patients is to induce and maintain long-term remission. Oral 5-aminosalicylate (5-ASA) plays an important role in both induction and maintenance therapies, and numerous studies have revealed that non-adherence to mesalazine is associated with an increased risk of clinical relapse [
2,
3]. Improving adherence to mesalazine is therefore an important goal in daily clinical practice.
Ethylcellulose-coated controlled-release (CR) mesalazine (PENTASA
®) is one of the major forms of oral 5-ASA [
4,
5]. CR mesalazine is available as 2 different formulations, tablets and granules, with no apparent difference in efficacy between them for UC, because both have the same mechanism of mesalazine release [
6]. However, the acceptabilities of the formulations may differ. In addition to the general differences between tablets and granules, CR mesalazine granules include a significantly lower percentage of additives compared with the tablets, while the numbers of tablets or sachets that need to be taken differ. It is therefore possible that these differences may influence patient adherence [
7,
8]; however, no study has yet compared these 2 formulations in terms of acceptability and adherence.
This study aimed to evaluate the acceptabilities of the 2 formulations of CR mesalazine in relation to adherence among UC patients, using a crossover questionnaire survey.
METHODS
1. Patients
Outpatients diagnosed with UC at Kitasato University Kitasato Institute Hospital or Kyorin University Hospital and who were eligible for CR mesalazine were recruited from January to December 2016 in Kitasato University Kitasato Institute Hospital, and from April to August 2017 in Kyorin University Hospital. There was no age limit as long as the patients could assess the acceptability of the medications and answer the questionnaires unaided.
2. Formulations of CR Mesalazine
This crossover study compared PENTASA® tablets and granules. PENTASA® tablets (250 mg, 500 mg, and 1 g) are approved in over 100 countries, and PENTASA® granules (250 mg, 500 mg, 1 g, 2 g, and 4 g) are approved in over 80 countries worldwide. A single tablet contains about 33% additives, and a total weight of 6 g is therefore needed to deliver 4 g mesalazine, compared with only 4.24 g of the granules. PENTASA® tablets (500 mg/tablet) and granules (2 g/sachet) were used in this study.
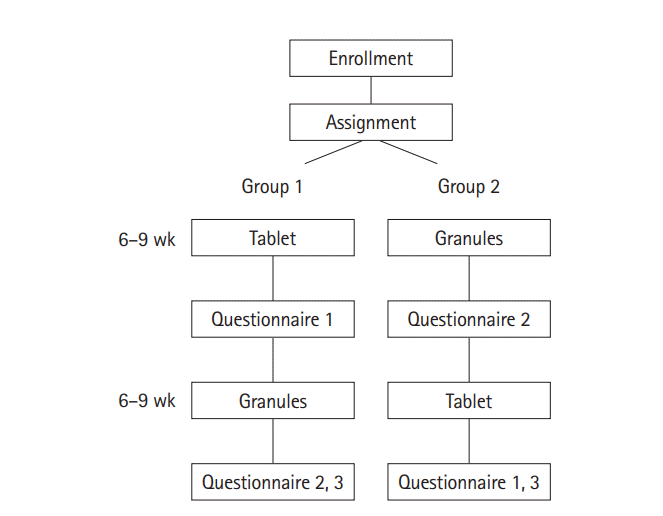
3. Study Design
The outline of the study is shown in
Fig. 1. Enrolled patients were randomly assigned to group 1 or group 2 in a 1:1 ratio. Patients in group 1 took CR mesalazine tablets and patients in group 2 took the granules for 6–9 (± 3) weeks, and each group then switched to the other formulation for a further 6–9 (±3) weeks. Patients who were administered 2 g twice daily were further evaluated to compare the acceptability, adherence, and efficacy of the formulations. The endpoints of the study were the acceptability, preference, adherence, efficacy, and safety of the 2 formulations. Acceptability and preference were assessed based on the answers to the questionnaires, efficacy was assessed based on the changes of the partial Mayo score before and after taking each formulation and based on the answers to the questionnaires. The differences between the 2 formulations were evaluated. Adherence rate (%) was assessed by a visual analog scale [
9], with an adherence rate of ≥80% defined as high adherence, and a rate of <80% defined as low adherence, as reported previously [
2,
3]. The average adherence rate, numbers of patients with high and low adherence, and adherence rate in each patient were compared between the 2 formulations. Safety was evaluated by assessing adverse events during the study period. The information on the enrolled patients was obtained from medical records.
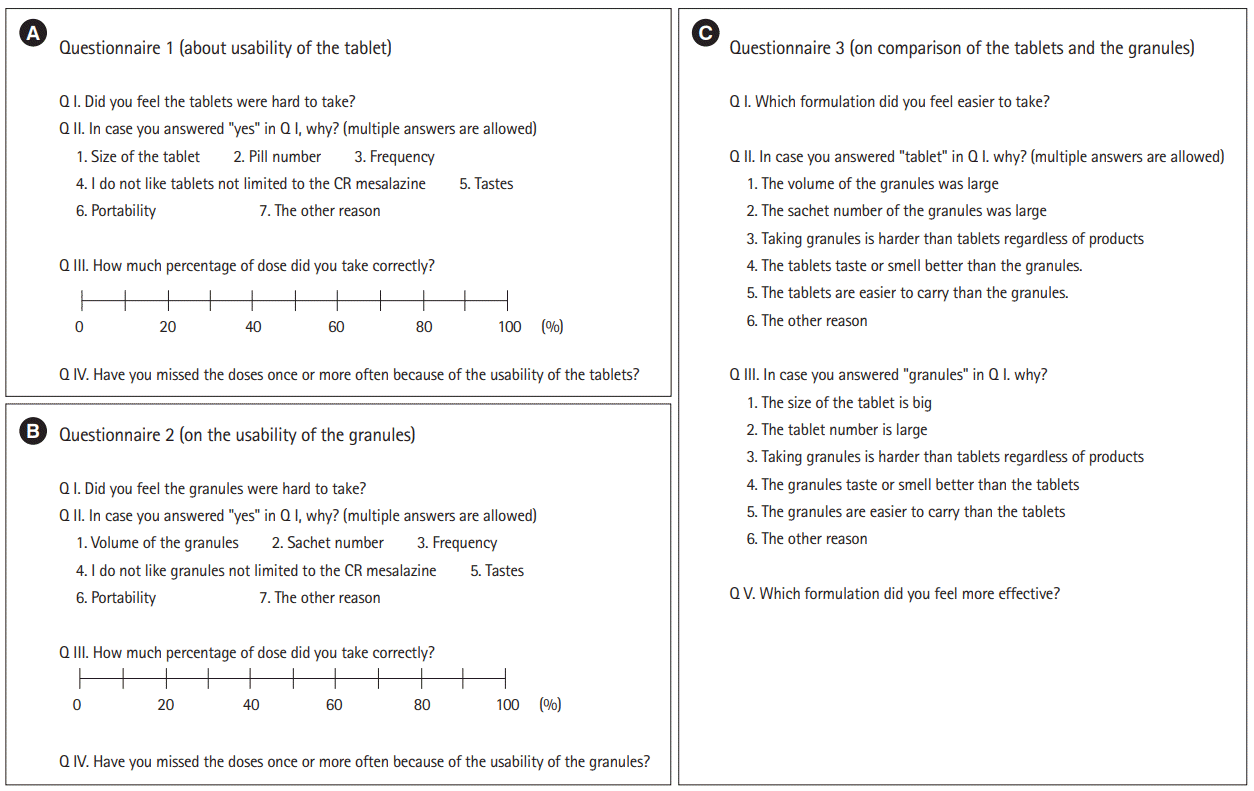
4. Questionnaires
The English versions of the questionnaires are shown in
Fig. 2 (the original was written in Japanese).
5. Statistical Analysis
All numerical values are shown as the median and range, or average ±SD. Continuous variables were compared by t-tests, and proportions of categorical variables were compared by chi-square and Fisher exact tests. A P-value ≤ 0.05 was considered statistically significant. Statistical analyses were performed using EZR version 1.33 (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
6. Ethical Considerations
This study was conducted in accordance with the Declaration of Helsinki and with good clinical practice. The study protocol was approved by the Institutional Review Boards of Kitasato University Kitasato Institute Hospital and Kyorin University School of Medicine. Written informed consent was obtained from all participating patients.
RESULTS
1. Patients
A total of 49 patients were prospectively enrolled. Twelve patients were excluded, including 11 who did not attend within the scheduled period and 1 who chose to withdraw from the study. Thirty-seven patients therefore completed the study. To ensure an accurate comparison of the acceptabilities and efficacies of the 2 formulations, 4 patients were excluded from the final analysis (33 patients) because they did not receive doses of 4 g/day, almost all patients were in remission (partial Mayo score, 1.0 ± 1.4) (
Table 1). There was no significant difference in any patient characteristics or questionnaire responses between the 2 groups, and we therefore assessed the acceptabilities of the 2 formulations without distinguishing between the groups.
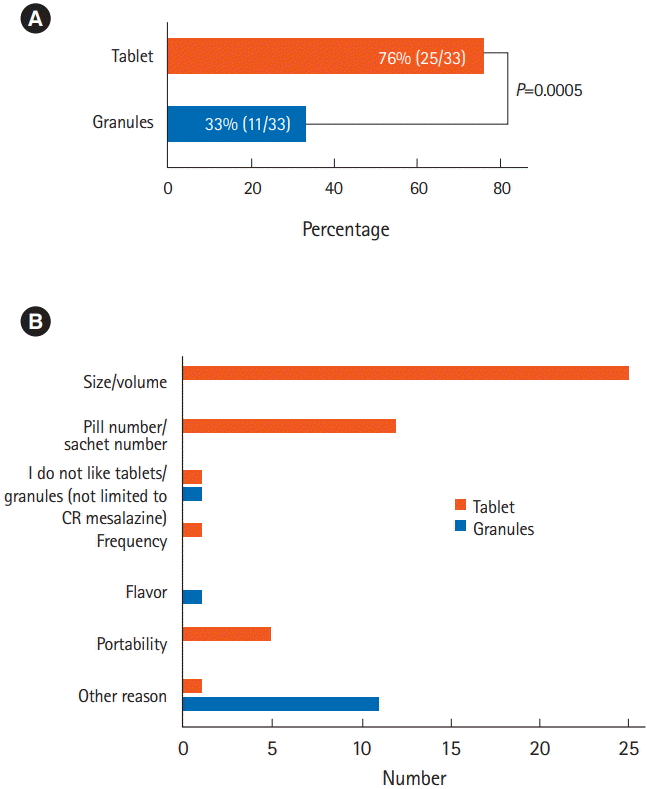
2. Formulation Acceptabilities
The results of questionnaires 1 and 2 (
Fig. 2) regarding the difficulties in taking each formulation are shown in
Fig. 3. Significantly more patients considered the tablets difficult to take compared with the granules (
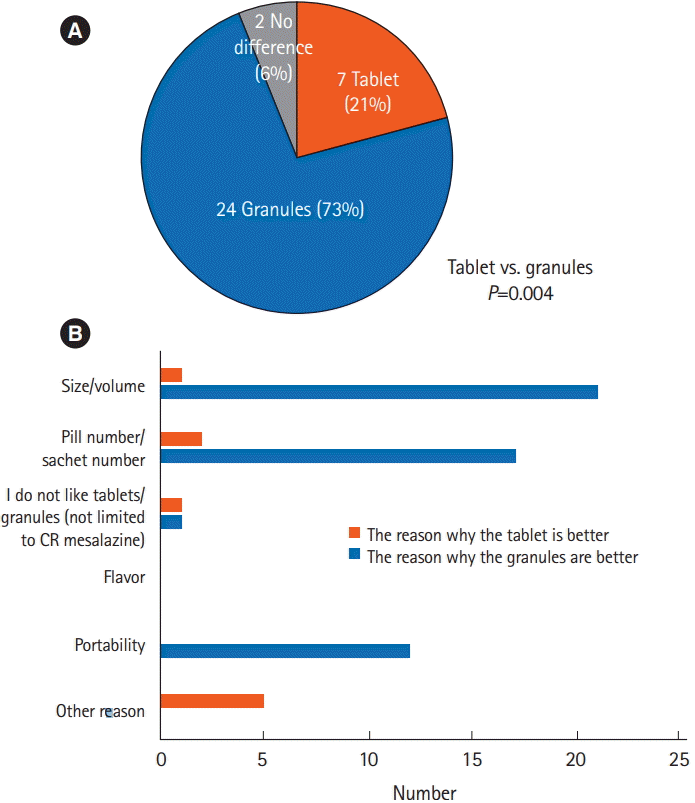
P=0.0005). When the 2 formulations were compared directly (questionnaire 3) (
Fig. 2C), patients considered the granules to be significantly more acceptable than the tablets (
P=0.004) (
Fig. 4A), largely due to the reduced volume of medication in the granule formulation (
Fig. 4B).
3. Medication Adherence
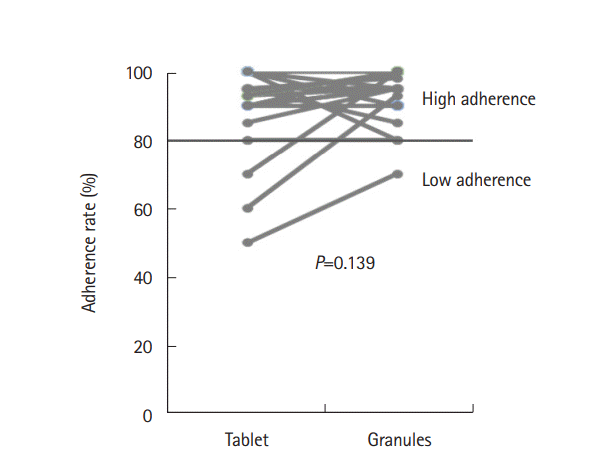
The adherence rate in patients taking the tablets was 91%±11% compared with 94% ± 8% in patients taking the granules (
Fig. 5). There was no significant difference between the adherence rates for the 2 formulations (
P=0.139), although adherence to the granules tended to be higher. Thirty percent of patients showed better adherence to the granules compared with the tablets, while 12% showed better adherence to the tablets (
P=0.180). Eighteen percent of patients missed taking tablets because of their acceptability, compared with only 3% who missed taking the granules (
P=0.0456). Eighteen percent also felt that the tablets were more likely to be missed than the granules, whereas no patient felt that the granules were more likely to be missed. Six percent of patients showed high adherence to the granules but low adherence to the tablets.
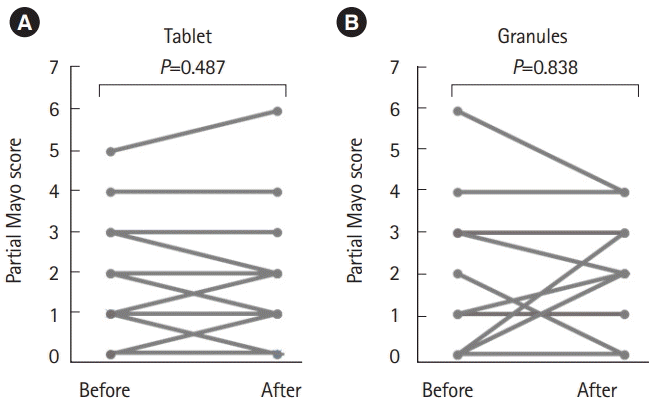
4. Efficacy
Before and after taking each formulation, no significant change of partial Mayo score was observed in both the 2 formulations (
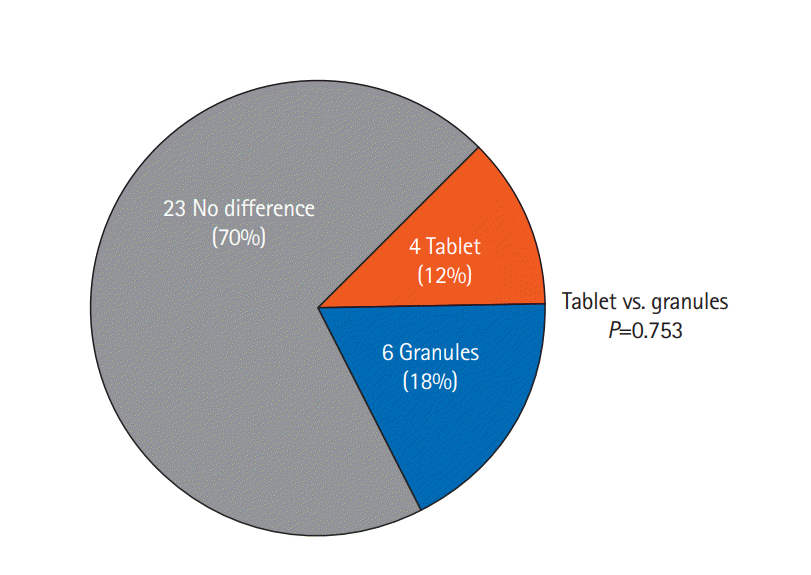
Fig. 6). The responses regarding the patients’ perceptions of formulation efficacy are shown in
Fig. 7. A total of 70% of patients noticed no difference between the 2 formulations, while 18% and 12% considered the granules and tablets to be more effective, respectively. There was no significant difference between the 2 formulations in terms of efficacy.
5. Adverse Events
Most patients experienced no adverse events related to CR mesalazine during the study period. One patient who had taken CR mesalazine tablets before enrollment experienced deterioration of abdominal symptoms during the granule period and improved after switching to the tablets; the partial Mayo score of the patient was 0 at enrollment, increased to 3 after taking the granules and decreased to 2 after taking the tablets.
DISCUSSION
To the best of our knowledge, this was the first study to compare the acceptability, adherence, and efficacy of 2 different formulations of CR mesalazine directly, using a crossover design to avoid potential bias. We demonstrated that CR mesalazine granules were more acceptable than tablets, and patient adherence tended to be slightly better for granules than for tablets, although there was no statistically significant difference.
The questionnaire responses revealed that many patients found the size and number of the tablets unpleasant. It is necessary to take 8 or 16 (500 or 250 mg, respectively) CR mesalazine tablets to take in 4 g of mesalazine. The situation is similar for other mesalazine formulations, such as pH-dependent mesalazine (Asacol®) (e.g., 10 tablets of 400 mg to administer 4 g mesalazine). Furthermore, the relatively large volume of CR mesalazine tablets, due to the large additive content, was also a major reason for the patient dissatisfaction. CR mesalazine granules were therefore preferable because of both their formulation and the reduced volume required.
The acceptability of the granules was significantly superior to that of the tablets, with approximately three-quarters of patients considering the granules to be preferable to the tablets by the end of this crossover study. Interestingly, the granule formulation was preferred not only because of the reduced pill burden in terms of volume and number, but also because of its portability. Medication portability may be an important factor for patients who need to take their medications outside their homes during their daily life, and patients may hesitate or forget to carry medications with them because of poor portability. Granules might thus be a good option for patients who take their medications outside their home.
Although the acceptabilities of the 2 formulations differed, their average adherence rates were not significantly different. The frequency of taking medications has been identified as one of the most important factors affecting adherence in patients with various diseases, including IBD [
10-
12]. In this study, the frequency of intake was the same for both formulations, which may help to explain why the average adherence rates were similar. However, the study design may have led to differences in adherence between the formulations being underestimated. It is difficult to assess real-world adherence in prospective studies, because patients may pay more attention to the protocol and/or overestimate their adherence under trial settings. In fact, the adherence rates in this study were very high (granules 95%, tablet 91%) compared with the previous reports [
2,
3]. In addition, we did not assess long-term adherence in this shortterm study, and it is possible that long-term adherence to the granules might be superior to that of the tablets in a real-world situation, because of significantly better acceptance.
The short-term nature of this study also limited the efficacy evaluation, and a longer observation period may be needed to assess the difference in efficacy in terms of maintaining remission. Adherence guidelines produced by the National Collaborating Centre for Primary Care suggest that although there is no convincing evidence that changes in drug formulation improve adherence, the number, taste, smell, size, and shape of the pills might nonetheless affect medication adherence [
13]. Kane et al. [
2] also reported that 30% of UC patients did not take their medication because of the large number of pills, and a lower adherence rate was associated with a higher risk of future clinical relapse. These findings suggest that adherence declines with lower acceptability of the medication in some patients, possibly leading to a flare-up. Indeed, 6% of patients in the current study showed high adherence to the granules but low adherence to the tablets, and all said that they had missed a dose at least once because of the poor acceptability of the tablets. Interestingly, a lower pill burden was also reported to be associated with better adherence and virological suppression in patients with human immunodeficiency virus infection, which also requires good adherence to the daily medication, and may be associated with treatment fatigue after longterm treatment [
14]. UC treatment has similar characteristics from the aspect of long-term maintenance, and the obvious difference in acceptability in the present study may thus have an important impact on the long-term treatment outcomes in patients with UC.
In conclusion, CR mesalazine granules are a highly acceptable formulation of 5-ASA, and may be associated with better long-term outcomes than tablets as a result of improved patient adherence to the medication.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download