Abstract
The Korean National Health Information Database (NHID) contains big data combining information obtained from the National Health Insurance Service and health examinations. Data are provided in the form of a cohort, and the NHID can be used to conduct longitudinal studies and research on rare diseases. Moreover, data on the cause and date of death are provided by Statistics Korea. Research and publications based on the NHID have increased explosively in the field of endocrine disorders. However, because the data were not collected for research purposes, studies using the NHID have limitations, particularly the need for the operational definition of diseases. In this review, we describe the characteristics of the Korean NHID, operational definitions of endocrine diseases used for research, and an overview of recent studies in endocrinology using the Korean NHID.
The Korean National Health Information Database (NHID) is actively used in health research, including the evaluation of the prevalence or incidence of diseases, the identification of disease risk factors, descriptions of treatment patterns or disease control status, and several fact sheets [1-5]. With the emerging importance of real-world evidence, its use has been increasing, especially in research on endocrine diseases. However, since the data were not collected for research purposes, studies using the NHID have limitations [1-3]. Caution is needed regarding operational definitions of diseases, covariates, or outcomes in studies. In this review, we present the characteristics of the Korean NHID, describe commonly used operational definitions of diseases, and discuss representative studies on endocrine diseases using the NHID.
The National Health Insurance Service (NHIS), a non-profit organization, is the single insurer that manages the health insurance system in Korea. Currently, approximately 97% of the Korean population are subscribers to the Korean NHIS [1-5]. The Health Insurance Review & Assessment Service (HIRA) evaluates the adequacy of healthcare service costs by reviewing medical billing and claims and announces the review results to the NHIS and healthcare service providers. Since 1995, the NHIS has provided general national health screening programs to improve Koreans’ health status through the prevention and early detection of diseases. In 2007, a health screening program for transitional ages, aimed at those aged 40 and 66 years, was launched. The general health screening program involves examinations at least once every 2 years for the entire population of Korean adults aged 40 years or older. General health screening for regional household members and dependents was recently expanded to include people aged 20 years or older. The NHIS established the Korean NHID in 2011, which incorporates all data from the NHIS and consists of five databases: an eligibility database, a national health screening database, a healthcare usage database, a long-term care insurance database, and a healthcare provider database [1-5]. Mortality data from Statistics Korea can be linked using resident registration numbers for wider application of the databases.
The NHID includes health screening information, containing detailed lifestyle questionnaires, laboratory results, and anthropometric measurements, which are not included in other claims databases [2]. Taiwan has a similar healthcare insurance system to that of Korea, and the National Health Research Institute collects registration and claims data of the entire population in Taiwan. However, Taiwan does not implement a health screening program; thus, the claims data do not include lifestyle variables, laboratory results, and anthropometric data [2]. The variables included in the health examinations and questionnaires have changed over time [3]. Currently, the parameters measured using blood tests include fasting blood glucose, total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol (calculated by the Friedewald formula), hemoglobin, creatinine, alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyl transferase (Supplemental Table S1) [3].
The database also contains data from the registration program for rare and intractable diseases (RIDs; V-code). Since 2006, the South Korean government has operated a RID registration program that includes 167 conditions [6,7]. Patients who meet the diagnostic criteria with physician certification are offered up to a 90% copayment reduction after registration in the RID program. Because the NHIS could refuse to pay hospital costs if the diagnosis does not meet specific criteria, cases are reviewed by medical institutions prior to submission to the NHIS, and the diagnoses can be presumed to be reliable [6,7]. The RID registration database has been used in several studies on the incidence and prevalence of various RIDs [6-8]. The Korean NHIS has implemented the RID registration program to support patients with these disease entities, enabling research on rare diseases, with a sufficient sample size that allows a better, more comprehensive understanding of the disease. Therefore, the data registered in the RID registry that are linked with the NHIS are verified and reliable.
The most important strength of the Korean NHID is the large number of individuals included in the database. In Korea, the NHIS is the single insurer managed by the government, and includes all Koreans. Thus, the NHID contains almost all medical use information that was registered during the claim process. As mentioned earlier, the NHID also includes health screening information, which is not included in other claims databases [2]. However, because the data were not collected for research purposes, studies using the NHID have limitations, particularly the need for operational definitions of diseases. There may also be a discrepancy between the real disease and the diagnosis claimed by healthcare providers—that is, in practice, diseases can be over- or under-diagnosed. To reduce inaccuracy, various methods can be used to improve operational definitions, such as limiting the diagnoses registered during admission, requiring repeated outpatient visits, incorporating the use of health screening results, integrating the data with prescription records, or using a special registration code for payment reduction [3].
The NHID does not include information about services that are not covered by the NHIS, such as cosmetic procedures, new technologies (such as robotic surgery), or uninsured new drugs [2].
Studies have used similar definitions, mostly based on the body mass index (BMI; body weight divided by height squared) and the waist circumference (WC) for abdominal obesity, according to obesity guidelines [9]. In the NHIS, data on BMI are available from 2006 onwards, but data for abdominal obesity have been available after 2009, when WC began to be measured [10]. General obesity has been defined as BMI ≥25 kg/m2 in adults, in accordance with the Asia-Pacific criteria of the World Health Organization guidelines and obesity guidelines by the Korean Society for the Study of Obesity (KSSO) [11]. Abdominal obesity has been defined as a WC ≥90 cm in men and ≥85 cm in women, in accordance with the definition by the KSSO [9].
The KSSO releases an Obesity Fact Sheet every 1 or 2 years, and the most recently updated Obesity Fact Sheet in 2021 contained results from the analysis of individuals ≥20 years old who underwent health checkups provided by the Korean NHIS between 2009 and 2019 [12]. The prevalence of general and abdominal obesity has increased in the entire population over the past 11 years, from 29.7% in 2009 to 36.3% in 2019 [12]. Obesity has risen in prevalence more rapidly in people in their 20s and 80s than in other age groups. The prevalence of abdominal obesity increased between 2009 and 2019 in both sexes, but most prominently in men (19.0% vs. 23.9% in the total population, 20.7% vs. 29.3% in men, and 16.2% vs. 19.0% in women). The relative risk of developing type 2 diabetes, myocardial infarction (MI), and ischemic stroke (IS) in people with obesity or abdominal obesity is greater than in people without obesity or abdominal obesity by 2- to 5-fold, depending on the age group. Another serious problem is that the prevalence of class III obesity in both men and women has significantly increased by nearly three-fold over the past 11 years, alerting stakeholders to the need for national interventions to combat this rising trend in higher-degree obesity.
Most studies using the NHID to investigate the relationship between obesity and cardiovascular disease (CVD) or mortality were initiated by the Taskforce Team of the Obesity Fact Sheet of the KSSO. In 11,524,763 Korean subjects older than age 20 years in whom NHIS health checkups were performed between 2005 and 2015, weight loss was associated with higher mortality rates than weight gain, with a 2.6-fold increase in mortality in individuals with ≥15% weight loss that was consistent across all BMI groups [13]. In addition, the highest mortality rates were found in individuals with a BMI ≥30 kg/m2, with a 3.5-fold increase in mortality. For abdominal obesity, an analysis of 23,263,878 participants in NHIS health checkups showed a linear association between WC and all-cause mortality across all BMI categories, and this association remained significant after adjustment for BMI, suggesting a deleterious effect of abdominal obesity on mortality risk [14].
This group also used the NHID to analyze the association between obesity and CVD. In a study of 11,084,683 participants in the Korean national health screening program from 2009 to 2012 who were followed up until 2015, the hazard ratio (HR) of IS was higher in those who experienced weight loss or weight gain than in those who maintained their weight. The risk showed a U-shaped curve, with those who maintained their weight showing the lowest risk, suggesting that the maintenance of body weight is an important determinant of the risk of IS [15]. Regarding abdominal obesity, the risk of MI and IS according to WC were analyzed in 21,749,261 participants in the national health screening program between 2009 and 2012 who were followed up until 2015 [16]. Analyses according to 11 groups of WC showed the lowest risk in subjects with WC of 70–74.9 and 65–69.9 cm in men and women, and the lowest risk of IS in subjects with WC of 65–69.9 and 60–64.9 cm in men and women, respectively. In addition, this analysis showed that WC had a better ability to predict CVD than BMI.
A recently published study evaluated the associations of BMI and WC with CVD in transitional ages—that is, those who are 40 and 66 years old [17]. This study was performed on 1,866,591 Korean adults who were 40 years of age and 563,919 adults who were 66 years of age, corresponding to these transitional stages, and participated in the national health screening program between 2009 and 2012. Among the participants aged 40 years, there was a J-shaped association of BMI with incident CVD, MI, and IS, with a nadir at a BMI of 18.6 to 22.9 kg/m2. Among those aged 66 years, there were significant U-shaped associations of BMI with CVD and MI, with a nadir at a BMI of 23.0 to 24.9 kg/m2. In contrast, WC showed linear association with all study outcomes in both age groups. This study suggested that the impact of general and abdominal obesity on CVD was more prominent in those aged 40 years than in those aged 66 years.
Heart failure (HF) is considered a forgotten or ignored complication of diabetes, and recent guidelines have focused on the prevention of HF as a highly important target of diabetes treatment [18]. Our group analyzed the risk of HF according to baseline glycemic status in 9,720,220 Koreans with a median follow-up of 6.3 years [19]. Participants with impaired fasting glucose (IFG) and diabetes showed 1.08- and 1.86-fold increased risk of HF, respectively, compared to normoglycemic participants. In addition, compared to normal-weight individuals, those who were underweight had a 1.7-fold increased risk of HF, while class II obesity was associated with a 1.1-fold increased risk of HF, suggesting a J-shaped association with BMI. This was the first study that showed an increased risk of HF among Koreans with IFG and demonstrated an association with obesity.
Another group analyzed the association between obesity and atrial fibrillation (AF). In 9,797,418 participants who underwent national health checkups, the risk for new-onset AF was analyzed according to the degree of obesity [20]. Obese, overweight, upper normal, and underweight participants showed significantly higher risks of new-onset AF than the reference group. In addition, a gradual escalation in the risk of new-onset AF was observed with more advanced stages of diabetes. Body weight and diabetic status had synergistic effects on the risk of new-onset AF.
In summary, these studies on obesity and CVD and mortality that analyzed NHID data showed that general obesity and abdominal obesity had significant deleterious effects on CVD and mortality from the standpoint of various disease categories.
Several studies have been published on the association between obesity and cancer using the NHID. For example, 139,519 men ≥40 years old who underwent national health examinations in 2002 to 2008 and did not have prostate cancer at baseline were followed up until 2012 [21]. The risk for prostate cancer according to BMI was analyzed separately according to the presence of diabetes. In patients without diabetes, the HR for prostate cancer significantly increased as BMI increased above the reference range, even with slight changes. In contrast, in patients with diabetes, the HR for prostate cancer significantly increased when BMI increased from <18.5 kg/m2 to within the reference range (18.5 to 22.9 kg/m2). In addition, a markedly lower HR for prostate cancer was observed in the population with a BMI <18.5 kg/m2 than in the reference or higher BMI group, suggesting that the risk for prostate cancer varies according to the change in BMI and the existence of diabetes [21,22]. Another study showed the additional effect of obesity on cancer risk [23] through an analysis of the relationship between BMI and bladder cancer risk using the NHID. In 23,378,895 participants in national health examinations between 2009 to 2012 without bladder cancer at baseline who were followed up until 2015, the risk for bladder cancer was lowest in people with a BMI <18.5 kg/m2 and highest among those with BMI ≥30 kg/m2, suggesting a significant relationship between BMI and bladder cancer risk [23]. Smoking also showed positive associations with bladder cancer risk, suggesting that obesity and smoking are significant determinants of bladder cancer. Another study showed an additional effect of abdominal obesity on bladder cancer risk [24].
An association between obesity and hepatocellular carcinoma (HCC) was documented in a study using NHID data for 10,505, 818 participants in national screening examinations in 2009 who were followed up for 7.3 years [25]. General obesity increased the risk of HCC, with HRs of 1.14 for the group with a BMI between 25 and 30 kg/m2 and 1.52 for those with a BMI ≥ 30 kg/m2 compared to participants with a BMI within the normal range. Central obesity, as assessed by WC, also showed a significant association with HCC risk. The authors concluded that the combination of general and central obesity particularly exacerbated HCC risk.
The economic burden of obesity-related cancer was analyzed using NHIS claims data during 2002 to 2015 [26]. Among the male obesity-related cancer sites, the greatest total costs of overweight or obesity were found for liver cancer, followed by colorectal cancer and kidney cancer. Among women, postmenopausal breast, liver, and colorectal cancers were the top three cancers with the highest total costs attributable to excess BMI. That study highlighted the importance of interventions for obesity both to improve health and to alleviate the economic medical burden of cancer in Korea.
Our group analyzed the risk of developing hypertension according to baseline WC in 16,312,476 participants without hypertension who received national health checkups from 2009 to 2012 [27]. The highest risk of incident hypertension was found among participants with the highest level of WC, using the middle group as the reference. Participants with abdominal obesity had a significantly higher HR for developing hypertension than in those without abdominal obesity; this relationship was independent of physical activity.
The association between pre-pregnancy WC and maternal complications in reproductive age was evaluated in a study using NHID data [28]. NHIS health checkup data from 280 to 645 days before childbirth of mothers of 783,406 deliveries from 2006 to 2015 were collected. BMI and WC showed positive associations with the incidence rate of maternal complications. The low-BMI and low-WC groups had higher odds of threatened abortion. To summarize, pre-pregnancy WC was closely linked to some maternal complications.
The association between abdominal obesity and fractures was investigated in an analysis of 1,556,751 participants who underwent NHIS health checkups between 2009 and 2011 and were followed up for 6.5 years. Higher WC was associated with a greater risk of femur fractures in both men and women [29]. Moreover, the incidence of lumbar fractures was also positively associated with WC in men and women, suggesting an association between abdominal obesity and fractures in Korean adults.
Lastly, the association between BMI and the risk of coronavirus disease 2019 (COVID-19) was analyzed using the NHID. A nationwide case-control study including 3,788 cases (patients confirmed to have COVID-19) and 15,152 age- and sex-matched controls, who were aged 20 years or more and underwent national health screening, was conducted using data from the NHIS with linkage to data from the Korea Disease Control and Prevention Agency [30]. Multivariate logistic regression models revealed a graded association between higher BMI and a higher risk of COVID-19 infection, and this association was robust across age and sex subgroups. Fig. 1 summarizes the effects of obesity and abdominal obesity on the risks of various diseases according to analyses of the Korean NHID.
Recent studies have suggested that variability in metabolic risk factors may be associated with mortality or CVD [31]. Several studies have used the NHID to investigate the associations of variability in body weight and BMI with the risk of mortality and chronic diseases such as CVD, diabetes, and dementia. In 125,391 participants in national health screening, variability in body weight and BMI was estimated as the standard deviation and coefficient of variation of serial measurements of BMI and body weight [32]. The highest quartile groups of the variability indices showed higher risks of all-cause mortality than the lowest quartiles after adjusting for confounding factors, suggesting that BMI and body weight variability may be independent risk factors for all-cause and cause-specific mortality. In another study performed in 4,244,460 participants in the national health screening program, compared to people in the lowest quartile of variability of body weight and WC, incrementally higher risks of IS and all-cause mortality were observed in individuals in higher quartiles of variability of body weight and WC [33].
The association between body weight fluctuations and the risk of type 2 diabetes was analyzed using NHID. In a total of 3,855,884 participants in NHIS health checkups, body weight variability was estimated using the average successive variability index [34]. Body weight fluctuation was associated with a higher risk of incident diabetes after adjustment for confounding variables, suggesting that body weight variability may be an independent risk factor for diabetes.
Lastly, the risk for dementia was analyzed in association with BMI and body weight changes in new-onset type 2 diabetes patients. In167,876 subjects aged ≥40 years diagnosed with newonset type 2 diabetes between 2007 and 2012, weight changes were monitored for up to 2 years after diagnosis, and the risks of all-cause dementia, Alzheimer’s disease, and vascular dementia were estimated [35]. The percentage of weight change during 2 years after a diagnosis of type 2 diabetes showed significant U-shaped associations with the risk of all-cause dementia development, and the risk increased significantly when the weight loss or gain was >10%. The results of that study suggest that weight loss or weight gain after the diagnosis of diabetes was associated with an increased risk of all-cause dementia in individuals with new-onset type 2 diabetes.
Thyroid diseases can be divided into two categories: anatomical diseases, such as thyroid nodules and cancer, and thyroid hormone dysfunction, which is mainly caused by autoimmune thyroid diseases. Research on thyroid diseases using big data in Korea has increased rapidly since 2012, and more than 60 research papers have been published as of 2022 (Fig. 2). These can also be categorized into the above two categories: thyroid nodules/cancer or thyroid dysfunction. The operational definitions for each specific thyroid disease are summarized in Table 1.
Approximately 45 papers using NHID data have been published in the field of thyroid nodules and cancer. First, several studies have aimed to identify risk factors related to the occurrence of thyroid cancer. In addition to clinical factors, such as metabolic syndrome, obesity, alcohol use, and smoking [36-46], the use of drugs known to have anti-cancer effects, such as aspirin [42], metformin [47], and statins [48], has shown associations with the occurrence of thyroid cancer. Associations between specific diseases and thyroid cancer have also been reported, especially in relation to sex differences, and patients with obstructive sleep apnea have also been shown to have a higher risk of incident thyroid cancer, especially in middle-aged men [49]. In addition, with the aim of explaining the predominance of thyroid cancer in women, a study investigated the effects of estrogen by analyzing whether the incidence of thyroid cancer decreased in women who underwent hysterectomy or bilateral salpingo-oophorectomy, and it obtained negative results [50]. That is, a sudden or early gradual decline in estrogen levels was not found to be a protective factor against the occurrence of thyroid cancer.
Second, a study investigated complications after thyroid cancer surgery and the long-term prognosis associated with postoperative hormone therapy or radioactive iodine (RAI) ablation treatment. Research on surgery-related complications has included studies on the occurrence of hypocalcemia and hypoparathyroidism [51,52], as well as studies related to cardiovascular risk or the risk of fracture during thyroid hormone suppression or supplementation therapy [53-55]. Interestingly, a study on the safety of RAI treatment reported that the incidence of leukemia significantly increased (HR, 3.1) in patients who received a cumulative dose of 100 mCi or more, and this has emerged as a major issue [56]. No association was reported between RAI treatment and the occurrence of long-term CVD [57]. In addition, studies on mortality [58,59] and second primary malignancies [60,61] were conducted to obtain insights into the long-term prognosis of thyroid cancer, and cases of simultaneous or sequential occurrence of thyroid cancer and breast cancer were reported [62]. Another study reported the diagnosis and treatment status of medullary thyroid carcinoma in Korea [63], and this report of the incidence and treatment status of a relatively rare disease furnishes a good example of big data utilization.
Among the big data studies in the field of thyroid dysfunction [64], the most important research results have been published by reports on complications related to antithyroid drug (ATD) use and the long-term prognosis of hyperthyroidism. Seo et al. [65] reported that ATD use in the first quarter of pregnancy was associated with the occurrence of congenital malformations, and in particular, a higher cumulative dose of methimazole (495 mg or more) was 1.87 times more dangerous than a low dose. Regarding the long-term prognosis of hyperthyroidism, increased risks for AF [66], HF [67], MI [68], and IS [68], accompanied by a consequent increase in mortality, have been reported, raising awareness of these issues among clinicians and patients. Interestingly, a study also reported that all-cause mortality was higher in patients with hypothyroidism receiving levothyroxine-treatment than in controls who were not diagnosed with hypothyroidism [69]. Two interesting reports regarding COVID-19 have recently been published. Ahn et al. [70] showed an increase in the incidence of subacute thyroiditis in 2020, when there was a COVID-19 outbreak in Korea, and since the spread of all other viral diseases that could cause this condition was significantly reduced at that time, the authors argued that the increase in subacute thyroiditis was related to COVID-19. In addition, a big data study reported that a past or present history of thyroid disease was not related to an elevated risk of mortality due to COVID-19 [71].
In conclusion, thyroid disease is a dynamic disease in which the pattern of disease occurrence changes and clinically unmet needs arise according to changes in various aspects of the environment, including epidemics of infectious diseases, environmental exposures, and medical technology. Therefore, active problem-solving efforts using smart big data are required.
In Korea, screening for osteoporosis is provided to women aged 54 and 66 years as part of the general health screening program. In a retrospective cohort study evaluating the 10-year fracture risk in postmenopausal women aged 66 years, osteoporosis was defined as a T-score of ≤–2.5 in bone mineral density, as suggested by the World Health Organization [72]. In other research using the NHID, osteoporosis patients who accessed medical services were operationally defined as those meeting at least one of the following six criteria: (1) prescription of medications used exclusively for osteoporosis treatment (bisphosphonates, selective estrogen receptor modulators, or calcitonin); (2) International Classification of Diseases, 10th revision (ICD-10) codes for osteoporosis (M80–M82) and the prescription of medications related to osteoporosis (hormones, calcium, or vitamin D); (3) elderly patients (men ≥70 years, women ≥65 years) with ICD-10 codes for osteoporosis; (4) a past history of prescribed medications known to cause osteoporosis along with the ICD-10 codes; (5) a past history of a disease known to cause osteoporosis along with the ICD-10 codes; (6) an osteoporosisrelated fracture (spine: M48.4, M48.5, S22.0, S22.1, S32.0; humerus: S42.2, S42.3; distal radius: S52.5, S52.6; hip: S72.0, S72.1). Each fracture code had to be accompanied by a physician’s claim for a site-specific procedure to enhance the specificity of the coding [73].
The presence of an osteoporotic fracture in the hip, vertebra, humerus, or distal radius was defined using ICD-10 diagnosis codes with or without site-specific procedure codes. In addition, radiographic study codes have also been used in some studies to improve the diagnostic accuracy of coding depending on the research purpose. The ICD-10 diagnosis codes for hip fractures were S72.0 (fracture of the femur neck) and S72.1 (trochanteric fracture), and the ICD-10 procedure codes for hip fracture included N0601/N0611 (open reduction), N0991 (closed pinning), N0981 (external fixation), N0715/N2710 (hemiarthroplasty), and N0711/N2070 (total joint arthroplasty) [74]. The operational definitions of vertebra, humerus, and distal radius fractures are given in Table 2 [74-77].
In a retrospective cohort study identifying the incidence, prognosis, and prognostic factors of primary hyperparathyroidism (PHPT) patients who underwent parathyroidectomy, PHPT was defined as when all of the following criteria were met: (1) at least two ICD-10 codes for PHPT (E210, E212, E213, or D351); (2) a procedure code for parathyroidectomy (P4541, P4542, or P4543); and (3) a history of admission [78]. In a retrospective cohort study estimating the prevalence of nonsurgical hypoparathyroidism and the risk of complications, nonsurgical hypoparathyroidism was defined as the presence of the ICD-10 codes (D82.1, E20.0, E20.8, E20.9, E31.0, E31.8, or E31.9) and at least two prescriptions for active vitamin D analogs. Subjects with a history of head and neck cancer, thyroid or parathyroid surgery, radiation to the neck region, or stage 5 chronic kidney disease were excluded [79].
Osteoporosis and Osteoporotic Fracture Fact Sheets were published in 2017, 2018, and 2019 based on the Korea National Health and Nutrition Examination Survey data and the NHID. Among Korean adults aged ≥50 years, the number of osteoporotic fractures in the hip, vertebra, humerus, and distal radius gradually increased from 2008 to 2016. However, the incidence of osteoporotic fractures plateaued in 2013 [80]. The fatality rate due to hip fractures was estimated to be 21.0% in men and 14.0% in women during the first 12 months. The fatality rate due to vertebral fractures was 9.0% in men and 4.0% in women. Among all osteoporosis patients in 2010, 61% accessed medical services and 33.5% were prescribed anti-osteoporosis medications. Only 21.0% of men and 48.2% of women with osteoporotic fractures took anti-osteoporosis medication in the first 12 months post-fracture. Although the prescription rate of anti-osteoporosis medications steadily increased by 6% from 2011 to 2016, the medication possession rates were only 33.2% and 21.5% at 12 and 24 months, respectively.
In the field of adrenal diseases, studies using the Korean NHID started with operational definitions of adrenal diseases. Operational definitions have been proposed for pheochromocytoma/paraganglioma (PPGL), adrenal Cushing syndrome (CS), congenital adrenal hyperplasia (CAH), and primary aldosteronism (PA), as shown in Table 3. Since the nationwide epidemiology of adrenal diseases in Korea had not been previously studied, researchers investigated the epidemiology of adrenal diseases based on operational definitions. The overall prevalence and age-standardized incidence rate of PPGL were 2.13 per 100,000 persons and 0.18 per 100,000 person-years [81]. The prevalence and age-standardized incidence rates of adrenal CS were 2.3 per 100,000 persons and 0.13 per 100,000 person-years [82]. Regarding CAH, the point prevalence in 2017 was 5.3 per 100,000 persons, and the annual incidence rate declined in recent years [83]. Research results have also been published on the complications and prognosis of adrenal diseases. According to Kim et al. [84], new-onset AF in patients with PA remained high for up to 3 years even after adrenalectomy or medical treatment compared to patients with essential hypertension. Ahn et al. [82] reported that adrenal CS was associated with a higher risk for mortality that was maintained for 10 years after adrenalectomy. This might be attributed to a significant residual risk for CVD even after biochemical remission. Kim et al. [81] showed that metastatic PPGLs accounted for 17.7% of all PPGLs. Intriguingly, among patients with initially non-metastatic PPGLs (n=954), 9.5% later presented with metastasis in a follow-up of around 6.5 years. This finding implied the need for long-term follow-up, even in PPGL patients without metastatic lesions. Compared with age-, sex-, and index-year-matched controls, patients with CAH harbored an elevated risk for CVD, stroke, diabetes mellitus, and psychiatric disorders [83]. That nationwide study yielded long-term outcome results across all age groups for rare congenital disorders.
Most patients with pituitary tumors are registered in the RID, which was initiated to provide financial support by reducing medical expenses for RIDs. Hence, the operational definitions of pituitary tumors based on ICD-10 and RID registration codes are relatively reliable. Among several pituitary diseases, studies have most actively focused on acromegaly [85-88]. Park et al. [85] first reported that the annual incidence rate of acromegaly in Korea was 0.36 cases per 100,000 persons. Interestingly, the authors found that malignancy and mortality risk was more pronounced in females than in males [85]. Hong et al. [87] investigated cardiovascular outcomes in patients with acromegaly. The risk for AF and congestive HF was higher in patients with acromegaly than in age- and sex-matched controls, but the risk for MI and stroke was similar between the two groups. In addition, the risk for AF was higher only in the first 4 years after diagnosis in patients with acromegaly. The same group also found higher risks for Parkinson’s disease and dementia in patients with acromegaly [88]. The nationwide epidemiology of other pituitary tumors was also evaluated. The annual incidence of prolactinoma and Cushing’s disease was 1.6–2.4 and 0.23 cases per 100,000 person-years [89-90]. In patients with Cushing’s disease, the mortality risk significantly decreased after treatment, but this effect of treatment on the mortality risk in patients with prolactinomas.
The NHID, which includes health examination information, is now extensively used in clinical and public health research, especially in the field of endocrine disorders. Careful research design and analysis of this source of big data can generate valuable information complementing other forms of research. With an understanding of the characteristics of the NHID and operational definitions of diseases used in previous studies, more research is expected to be conducted in the future, and it is hoped that this research will lead to actual initiatives for health promotion.
Supplementary material
Supplementary Table 1.
Health Screening Items and Target Diseases in the Health Examination Database of the Korean National Health Information Database
REFERENCES
1. Kim MK, Han K, Lee SH. Current trends of big data research using the Korean National Health Information Database. Diabetes Metab J. 2022; 46:552–63.

2. Choi EK. Cardiovascular research using the Korean National Health Information Database. Korean Circ J. 2020; 50:754–72.

3. Shin DW, Cho J, Park JH, Cho B. National General Health Screening Program in Korea: history, current status, and future direction. Precis Future Med. 2022; 6:9–31.

4. Kim HK, Song SO, Noh J, Jeong IK, Lee BW. Data configuration and publication trends for the Korean National Health Insurance and Health Insurance Review & Assessment Database. Diabetes Metab J. 2020; 44:671–8.

5. National Health Insurance Service. 2021 National Health Insurance statistical yearbook [Internet]. Wonju: National Health Insurance Service;2022. [cited 2023 Jan 29]. Available from: https://www.nhis.or.kr/nhis/together/wbhaec06300m01.do?mode=view&articleNo=10829400&article.offset=0&articleLimit=10.
6. Won JH, Byun SJ, Oh BM, Park SJ, Seo HG. Risk and mortality of aspiration pneumonia in Parkinson’s disease: a nationwide database study. Sci Rep. 2021; 11:6597.

7. Yang MS, Park M, Back JH, Lee GH, Shin JH, Kim K, et al. Validation of cancer diagnosis based on the National Health Insurance Service Database versus the National Cancer Registry Database in Korea. Cancer Res Treat. 2022; 54:352–61.

8. Lee HR, Yoo JE, Choi H, Han K, Jung JH, Park J, et al. Tuberculosis and risk of ischemic stroke: a nationwide cohort study. Stroke. 2022; 53:3401–9.

9. Kim BY, Kang SM, Kang JH, Kang SY, Kim KK, Kim KB, et al. 2020 Korean Society for the Study of Obesity guidelines for the management of obesity in Korea. J Obes Metab Syndr. 2021; 30:81–92.

10. Kim YH, Han K, Son JW, Lee SS, Oh SW, Kwon HS, et al. Data analytic process of a nationwide population-based study on obesity using the National Health Information Database presented by the National Health Insurance Service 2006-2015. J Obes Metab Syndr. 2017; 26:23–7.

11. World Health Organization; Regional Office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia;2000.
12. Yang YS, Han BD, Han K, Jung JH, Son JW; Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Obesity fact sheet in Korea, 2021: trends in obesity prevalence and obesity-related comorbidity incidence stratified by age from 2009 to 2019. J Obes Metab Syndr. 2022; 31:169–77.

13. Kim YH, Kim SM, Han KD, Son JW, Lee SS, Oh SW, et al. Change in weight and body mass index associated with allcause mortality in Korea: a nationwide longitudinal study. J Clin Endocrinol Metab. 2017; 102:4041–50.

14. Kim YH, Kim SM, Han KD, Jung JH, Lee SS, Oh SW, et al. Waist circumference and all-cause mortality independent of body mass index in Korean population from the National Health Insurance Health Checkup 2009-2015. J Clin Med. 2019; 8:72.

15. Cho JH, Rhee EJ, Park SE, Kwon H, Jung JH, Han KD, et al. Maintenance of body weight is an important determinant for the risk of ischemic stroke: a nationwide populationbased cohort study. PLoS One. 2019; 14:e0210153.

16. Cho JH, Rhee EJ, Park SE, Kwon H, Jung JH, Han KD, et al. The risk of myocardial infarction and ischemic stroke according to waist circumference in 21,749,261 Korean adults: a nationwide population-based study. Diabetes Metab J. 2019; 43:206–21.

17. Yoo JE, Han K, Jung JH, Hur YI, Kim YH, Kim ES, et al. Body mass index, waist circumference and cardiovascular diseases in transitional ages (40 and 66 years). J Cachexia Sarcopenia Muscle. 2022 Dec 15 [Epub]. https://doi.org/10.1002/jcsm.13138.

18. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023; 46(Suppl 1):S140–57.
19. Rhee EJ, Kwon H, Park SE, Han KD, Park YG, Kim YH, et al. Associations among obesity degree, glycemic status, and risk of heart failure in 9,720,220 Korean adults. Diabetes Metab J. 2020; 44:592–601.

20. Kim YG, Han KD, Choi JI, Boo KY, Kim DY, Oh SK, et al. The impact of body weight and diabetes on new-onset atrial fibrillation: a nationwide population based study. Cardiovasc Diabetol. 2019; 18:128.

21. Choi JB, Moon HW, Park YH, Bae WJ, Cho HJ, Hong SH, et al. The impact of diabetes on the risk of prostate cancer development according to body mass index: a 10-year nationwide cohort study. J Cancer. 2016; 7:2061–6.

22. Choi JB, Myong JP, Lee Y, Kim I, Kim JH, Hong SH, et al. Does increased body mass index lead to elevated prostate cancer risk?: it depends on waist circumference. BMC Cancer. 2020; 20:589.

23. Choi JB, Lee EJ, Han KD, Hong SH, Ha US. Estimating the impact of body mass index on bladder cancer risk: stratification by smoking status. Sci Rep. 2018; 8:947.

24. Choi JB, Kim JH, Hong SH, Han KD, Ha US. Association of body mass index with bladder cancer risk in men depends on abdominal obesity. World J Urol. 2019; 37:2393–400.

25. Hwang S, Park YM, Han KD, Yun JS, Ko SH, Ahn YB, et al. Associations of general obesity and central obesity with the risk of hepatocellular carcinoma in a Korean population: a national population-based cohort study. Int J Cancer. 2021; 148:1144–54.
26. Lee JE, Nam CM, Lee SG, Park S, Kim TH, Park EC. The economic burden of cancer attributable to obesity in Korea: a population-based cohort study. Eur J Cancer Care (Engl). 2019; 28:e13084.

27. Rhee EJ, Cho JH, Kwon H, Park SE, Jung JH, Han KD, et al. Association between abdominal obesity and increased risk for the development of hypertension regardless of physical activity: a nationwide population-based study. J Clin Hypertens (Greenwich). 2018; 20:1417–26.

28. Lim J, Han K, Kim SY, Cho YH, Yoon YS, Park HS, et al. Effects of central obesity on maternal complications in Korean women of reproductive age. Obes Res Clin Pract. 2019; 13:156–63.

29. Park GR, Kim HS, Kim YT, Chung HJ, Ha SJ, Kim DW, et al. Waist circumference and the risk of lumbar and femur fractures: a nationwide population-based cohort study. Eur Rev Med Pharmacol Sci. 2021; 25:1198–205.
30. Jung CY, Park H, Kim DW, Lim H, Chang JH, Choi YJ, et al. Association between body mass index and risk of coronavirus disease 2019 (COVID-19): a nationwide case-control study in South Korea. Clin Infect Dis. 2021; 73:e1855–62.

31. Lee SH, Kim MK, Rhee EJ. Effects of cardiovascular risk factor variability on health outcomes. Endocrinol Metab (Seoul). 2020; 35:217–26.

32. Nam GE, Cho KH, Han K, Han B, Cho SJ, Roh YK, et al. Impact of body mass index and body weight variabilities on mortality: a nationwide cohort study. Int J Obes (Lond). 2019; 43:412–23.

33. Kim DH, Nam GE, Han K, Kim YH, Park KY, Hwang HS, et al. Variabilities in weight and waist circumference and risk of myocardial infarction, stroke, and mortality: a nationwide cohort study. Endocrinol Metab (Seoul). 2020; 35:933–42.

34. Park KY, Hwang HS, Cho KH, Han K, Nam GE, Kim YH, et al. Body weight fluctuation as a risk factor for type 2 diabetes: results from a nationwide cohort study. J Clin Med. 2019; 8:950.

35. Nam GE, Park YG, Han K, Kim MK, Koh ES, Kim ES, et al. BMI, weight change, and dementia risk in patients with new-onset type 2 diabetes: a nationwide cohort study. Diabetes Care. 2019; 42:1217–24.

36. Yeo Y, Shin DW, Han K, Kim D, Kim TH, Chun S, et al. Smoking, alcohol consumption, and the risk of thyroid cancer: a population-based Korean cohort study of 10 million people. Thyroid. 2022; 32:440–8.

37. Song Y, Lee HS, Park G, Kang SW, Lee JW. Dyslipidemia risk in thyroid cancer patients: a nationwide populationbased cohort study. Front Endocrinol (Lausanne). 2022; 13:893461.

38. Park JH, Cho HS, Yoon JH. Thyroid cancer in patients with metabolic syndrome or its components: a nationwide population-based cohort study. Cancers (Basel). 2022; 14:4106.

39. Kim J, Kim MK, Baek KH, Song KH, Han K, Kwon HS. Repeated low high-density lipoprotein cholesterol and the risk of thyroid cancer: a nationwide population-based study in Korea. Endocrinol Metab (Seoul). 2022; 37:303–11.
40. Park JH, Choi M, Kim JH, Kim J, Han K, Kim B, et al. Metabolic syndrome and the risk of thyroid cancer: a nationwide population-based cohort study. Thyroid. 2020; 30:1496–504.

41. An SY, Kim SY, Oh DJ, Min C, Sim S, Choi HG. Obesity is positively related and tobacco smoking and alcohol consumption are negatively related to an increased risk of thyroid cancer. Sci Rep. 2020; 10:19279.

42. Lee YK, Hwangbo Y, Lee S, Lee DE, Lee EK, Yeom MS, et al. Aspirin use is not associated with lower thyroid cancer risk: a nationwide nested case-control study. Thyroid. 2020; 30:829–37.

43. Yeo Y, Han K, Shin DW, Kim D, Jeong SM, Chun S, et al. Changes in smoking, alcohol consumption, and the risk of thyroid cancer: a population-based Korean cohort study. Cancers (Basel). 2021; 13:2343.

44. Kim ES, Han K, Kwon HS. The association between metabolic syndrome, obesity phenotype, and the risk of thyroid cancer. Diabetes. 2019; 68(Supplement_1):2107-P.
45. Son H, Lee H, Kang K, Lee I. The risk of thyroid cancer and obesity: a nationwide population-based study using the Korea National Health Insurance Corporation cohort database. Surg Oncol. 2018; 27:166–71.

46. An J, Kim T, Kim N, Kim H, Choi D, Park Y, et al. Demographic and clinical risk factors for thyroid cancer in Korea: Korean National Health Insurance Database study. Thyroid. 2015; 25(Supp 1):A114–5.
47. Cho YY, Kang MJ, Kim SK, Jung JH, Hahm JR, Kim TH, et al. Protective effect of metformin against thyroid cancer development: a population-based study in Korea. Thyroid. 2018; 28:864–70.

48. Kim SY, Song YS, Wee JH, Min C, Yoo DM, Lee CH, et al. Evaluation of the relationship between previous statin use and thyroid cancer using Korean National Health Insurance Service-Health Screening Cohort data. Sci Rep. 2021; 11:7912.

49. Choi JH, Lee JY, Lim YC, Kim JK, Han KD, Cho JH. Association between obstructive sleep apnea and thyroid cancer incidence: a national health insurance data study. Eur Arch Otorhinolaryngol. 2021; 278:4569–74.

50. Kim M, Kim BH, Lee H, Nam H, Park S, Jang MH, et al. Thyroid cancer after hysterectomy and oophorectomy: a nationwide cohort study. Eur J Endocrinol. 2021; 184:143–51.

51. Hyun MK, Kim J, Kwon JW, Park YJ. PSU2 The incidence of complications after its thyroidectomy in patients with thyroid cancer in Korea: using health claim database. Value Health. 2012; 15:A402.
52. Jee E, Park SY, Ahn SV, Lee S. Incidence and prevalence of post-surgical hypoparathyroidism in Korea. J Bone Miner Res. 2017; 32:S120.
53. Suh B, Shin DW, Park Y, Lim H, Yun JM, Song SO, et al. Increased cardiovascular risk in thyroid cancer patients taking levothyroxine: a nationwide cohort study in Korea. Eur J Endocrinol. 2019; 180:11–20.

54. Shin DW, Suh B, Lim H, Yun JM, Song SO, Park Y. Jshaped association between postoperative levothyroxine dosage and fracture risk in thyroid cancer patients: a retrospective cohort study. J Bone Miner Res. 2018; 33:1037–43.

55. Shin DW, Suh B, Yoon JM, Park Y. Risk of coronary heart disease and ischemic stroke in thyroid cancer patients taking levothyroxine. J Clin Oncol. 2017; 35(5_suppl):105.

56. Seo GH, Cho YY, Chung JH, Kim SW. Increased risk of leukemia after radioactive iodine therapy in patients with thyroid cancer: a nationwide, population-based study in Korea. Thyroid. 2015; 25:927–34.

57. Kim KJ, Song JE, Kim JY, Bae JH, Kim NH, Yoo HJ, et al. Effects of radioactive iodine treatment on cardiovascular disease in thyroid cancer patients: a nationwide cohort study. Ann Transl Med. 2020; 8:1235.

58. Kim KJ, Choi J, Kim JY, Kim NH, Kim HY, Baek SK, et al. The paradox of decreased all-cause mortality in patients with thyroid cancer compared to general population in Korea: a nationwide case-control cohort study. Thyroid. 2022; 32(Suppl 1):A61.
59. Kim KJ, Jang S, Kim KJ, An JH, Kim NH, Shin DY, et al. Actual causes of death in thyroid cancer patients in Korea: a nationwide case control cohort study. Eur J Endocrinol. 2020; 182:103–10.

60. Kim M, Kim H, Park S, Joo J, Kim IJ, Kim BH. Risk factors for second primary malignancies following thyroid cancer: a nationwide cohort study. Eur J Endocrinol. 2022; 186:561–71.

61. Kim KJ, Choi J, Kim JY, Kim KJ, Bae JH, Kim JH, et al. Increased risk of second primary malignancy in patients with thyroid cancer: is it a real phenomenon or a detection bias? Thyroid. 2021; 31(Suppl 1):A24.
62. Jin YJ, Kwon MJ, Kim JH, Kim JH, Choi HG. Association between thyroid cancer and breast cancer: two longitudinal follow-up studies using a national health screening cohort. J Pers Med. 2022; 12:133.

63. Ahn HY, Chae JE, Moon H, Noh J, Park YJ, Kim SG. Trends in the diagnosis and treatment of patients with medullary thyroid carcinoma in Korea. Endocrinol Metab (Seoul). 2020; 35:811–9.

64. Choi HG, Kim TJ, Hong SK, Min C, Yoo DM, Kim H, et al. Thyroid diseases and chronic rhinosinusitis: a nested casecontrol study using a national health screening cohort. Int J Environ Res Public Health. 2022; 19:8372.

65. Seo GH, Kim TH, Chung JH. Antithyroid drugs and congenital malformations: a nationwide Korean cohort study. Ann Intern Med. 2018; 168:405–13.
66. Cho YY, Kim B, Choi D, Kim CH, Shin DW, Kim JS, et al. Graves’ disease, its treatments, and the risk of atrial fibrillation: a Korean population-based study. Front Endocrinol (Lausanne). 2022; 13:1032764.

67. Song E, Kim M, Park S, Park MJ, Kim JA, Roh E, et al. Treatment modality and risk of heart failure in patients with long-standing Graves’ disease: a nationwide populationbased cohort study. Front Endocrinol (Lausanne). 2021; 12:761782.

68. Kim HJ, Kang T, Kang MJ, Ahn HS, Sohn SY. Incidence and mortality of myocardial infarction and stroke in patients with hyperthyroidism: a nationwide cohort study in Korea. Thyroid. 2020; 30:955–65.

69. Sohn SY, Seo GH, Chung JH. Risk of all-cause mortality in levothyroxine-treated hypothyroid patients: a nationwide Korean cohort study. Front Endocrinol (Lausanne). 2021; 12:680647.

70. Ahn HY, Choi HS, Ha S, Cho SW. Incidence of subacute thyroiditis during the COVID-19 pandemic in South Korea using the National Health Insurance Service Database. Thyroid. 2022; 32:1299–306.

71. Kim SY, Yoo DM, Min CY, Choi HG. The effects of previous thyroid disease on the susceptibility to, morbidity of, and mortality due to COVID-19: a nationwide cohort study in South Korea. J Clin Med. 2021; 10:3522.

72. Baek YH, Cho SW, Jeong HE, Kim JH, Hwang Y, Lange JL, et al. 10-Year fracture risk in postmenopausal women with osteopenia and osteoporosis in South Korea. Endocrinol Metab (Seoul). 2021; 36:1178–88.

73. Yu TY, Cho H, Kim TY, Ha YC, Jang S, Kim HY. Utilization of osteoporosis-related health services: use of data from the Korean National Health Insurance Database 2008-2012. J Korean Med Sci. 2018; 33:e20.

74. Lee YK, Yoo JI, Kim TY, Ha YC, Koo KH, Choi H, et al. Validation of operational definition to identify patients with osteoporotic hip fractures in administrative claims data. Healthcare (Basel). 2022; 10:1724.

75. Park SM, Ahn SH, Kim HY, Jang S, Ha YC, Lee YK, et al. Incidence and mortality of subsequent vertebral fractures: analysis of claims data of the Korea National Health Insurance Service from 2007 to 2016. Spine J. 2020; 20:225–33.

76. Jung HS, Nho JH, Ha YC, Jang S, Kim HY, Yoo JI, et al. Incidence of osteoporotic refractures following proximal humerus fractures in adults aged 50 years and older in Korea. J Bone Metab. 2019; 26:105–11.

77. Kwon GD, Jang S, Lee A, Park CM, Lee YK, Kim TY, et al. Incidence and mortality after distal radius fractures in adults aged 50 years and older in Korea. J Korean Med Sci. 2016; 31:630–4.

78. Kong SH, Kim JH, Park MY, Kim SW, Shin CS. Residual risks of comorbidities after parathyroidectomy in a nationwide cohort of patients with primary hyperparathyroidism. Endocrine. 2023; 79:190–9.

79. Kim SH, Rhee Y, Kim YM, Won YJ, Noh J, Moon H, et al. Prevalence and complications of nonsurgical hypoparathyroidism in Korea: a nationwide cohort study. PLoS One. 2020; 15:e0232842.

80. Ahn SH, Park SM, Park SY, Yoo JI, Jung HS, Nho JH, et al. Osteoporosis and osteoporotic fracture fact sheet in Korea. J Bone Metab. 2020; 27:281–90.

81. Kim JH, Moon H, Noh J, Lee J, Kim SG. Epidemiology and prognosis of pheochromocytoma/paraganglioma in Korea: a nationwide study based on the National Health Insurance Service. Endocrinol Metab (Seoul). 2020; 35:157–64.

82. Ahn CH, Kim JH, Park MY, Kim SW. Epidemiology and comorbidity of adrenal Cushing syndrome: a nationwide cohort study. J Clin Endocrinol Metab. 2021; 106:e1362–72.

83. Kim JH, Choi S, Lee YA, Lee J, Kim SG. Epidemiology and long-term adverse outcomes in Korean patients with congenital adrenal hyperplasia: a nationwide study. Endocrinol Metab (Seoul). 2022; 37:138–47.

84. Kim KJ, Hong N, Yu MH, Lee H, Lee S, Lim JS, et al. Time-dependent risk of atrial fibrillation in patients with primary aldosteronism after medical or surgical treatment initiation. Hypertension. 2021; 77:1964–73.

85. Park KH, Lee EJ, Seo GH, Ku CR. Risk for acromegaly-related comorbidities by sex in Korean acromegaly. J Clin Endocrinol Metab. 2020; 105:dgz317.

86. Yun SJ, Lee JK, Park SY, Chin SO. Descriptive epidemiology and survival analysis of acromegaly in Korea. J Korean Med Sci. 2021; 36:e159.

87. Hong S, Kim KS, Han K, Park CY. Acromegaly and cardiovascular outcomes: a cohort study. Eur Heart J. 2022; 43:1491–9.

88. Hong S, Han K, Kim KS, Park CY. Risk of neurodegenerative diseases in patients with acromegaly: a cohort study. Neurology. 2022; 99:e1875–85.
Fig. 1.
Effects of obesity and abdominal obesity on the risks of other diseases according to studies analyzing the Korean National Health Information Database. ↑ depicts a linear increase in the risk, U depicts an increase in the risk both in low and high body mass index groups. COVID-19, coronavirus disease 2019.
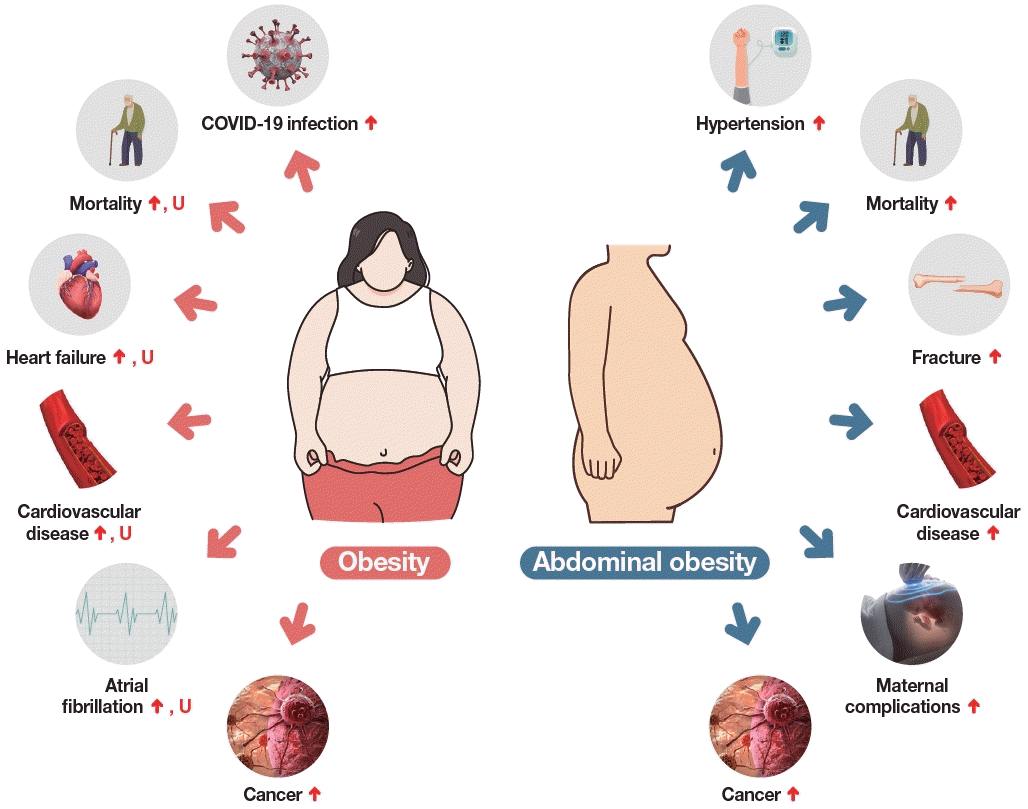
Fig. 2.
The number of articles about thyroid disease using Korean nationwide big data according to the year of publication.
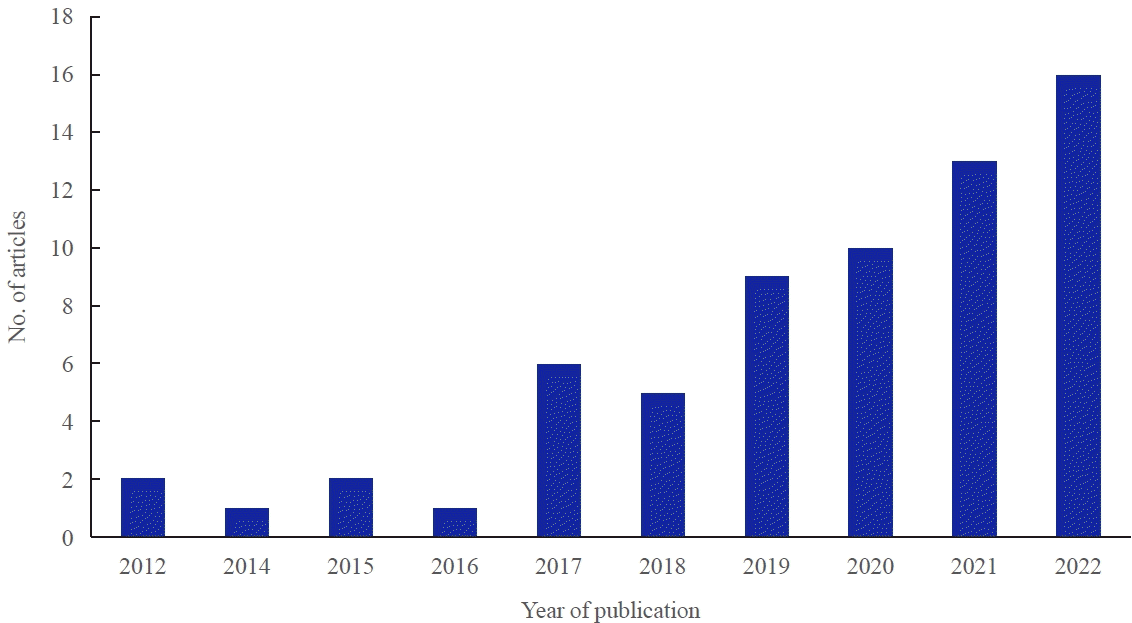
Table 1.
Operational Definitions of Thyroid Disease
| Disease | ICD-10 code | Additional definitions | Reference | |
|---|---|---|---|---|
| Thyroid cancer | ||||
| All cancer | C73 | Thyroidectomy (P4551-4, P4561) or special copayment reduction program code (V193, V194) | [36] | |
| Medullary thyroid cancer | C73 | Excluding ≥2 measurements of serum thyroglobulin and including ≥2 measurements of serum calcitonin | [63] | |
| Functional disease | ||||
| Hypothyroidism | E02, E03 | Prescription of levothyroxine ≥2 times or 180 days | [64] | |
| Graves’ disease | E05 | Prescription of antithyroid drugs ≥60 or 90 or 180 days | [64] | |
| Subacute thyroiditis | E061 | Two or more E061 codes and erythrocyte sedimentation rate test ≥1 time | [70] | |
Table 2.
Operational Definitions of Commonly Used Outcomes and Covariates in the Field of Bone Metabolism and Fracture Research
| ICD-10 codes and additional definitions | General health checkup results | Reference | |
|---|---|---|---|
| Osteoporosis or osteoporosis patients | At least one of the following six criteria: | T-score of ≤–2.5 for bone mineral density | [72] |
| (1) prescription of medications used exclusively for osteoporosis treatment (bisphosphonates, selective estrogen receptor modulators, or calcitonin); (2) ICD-10 codes for osteoporosis (M80–M82) and prescription of medications related to osteoporosis (hormones, calcium, or vitamin D); (3) elderly patients (men ≥70 years, women ≥65 years) with an ICD-10 codes for osteoporosis; (4) a past history of prescribed medications known to cause osteoporosis along with the ICD-10 codes; (5) a past history of a disease known to cause osteoporosis along with the ICD-10 codes; (6) osteoporosis-related fracture (spine: M48.4, M48.5, S22.0, S22.1, S32.0; humerus: S42.2, S42.3; distal radius: S52.5, S52.6; hip: S72.0, S72.1). Each fracture code had to be accompanied by a physician’s claim for site-specific procedure to enhance the specificity of the coding. | |||
| Hip fracture | ICD-10 diagnosis codes (S72.0, S72.1) with or without ICD-10 procedure codes (N0601, N0611, N0991, N0981, N0715, N2710, N0711, N2070) | [74] | |
| Vertebra fracture | ICD-10 diagnosis codes (S22.0, S22.1, S32.0, S32.7, T08, M48.4, M48.5, M49.5, M80.88) with or without ICD-10 procedure codes (N0471, N0472, N0473, N0474, N0630) | [75] | |
| Humerus fracture | ICD-10 diagnosis codes (S42.2, S42.3) with or without ICD-10 procedure codes (N0602, N0612, N0992, N0982, N0986, N0722, N2711, N2716, N0271, N0276, T6010, T6110) | [76] | |
| Distal radius fracture | ICD-10 diagnosis codes (S52.5, S52.6) with or without ICD-10 procedure codes (N0603, N0607, N0993, N0994, N0983, N0643, T6020, T6030) | [77] | |
| Hyperparathyroidism | All of the following criteria: | [78] | |
| (1) at least two ICD-10 codes (E210, E212, E213, or D351); (2) procedure code for parathyroidectomy (P4541, P4542, or P4543); (3) history of admission | |||
| Hypoparathyroidism (nonsurgical) | ICD-10 codes (D82.1, E20.0, E20.8, E20.9, E31.0, E31.8, or E31.9) and at least two prescriptions for active vitamin D analogs | [79] |
Table 3.
Operational Definitions of Adrenal and Pituitary Diseases
|
ICD-10 diagnostic code |
Procedural/treatment code |
Reference | |||
|---|---|---|---|---|---|
| Inclusion | Exclusion | Inclusion | Exclusion | ||
| Pheochromocytoma/Paraganglioma | ≥2 D350, D441, I1522, C741, or C749; D356, D446, D447, D487, or C755 (primary diagnosis) | E260, EI1520, I1521, E240, E248, E249, C740 (primary or secondary diagnosis) | P4571, P4572, P4581, P4582, Q2501, Q2502, R3512, Q1591, Q1592 | [81] | |
| Biochemical tests for catecholamine excess at least twice, including at least one preoperative biochemical test | |||||
| (≥2 C3211, C3212, C3213, C3231, C3232, C3233, C3234, C3235, C3239) | |||||
| Adrenal Cushing syndrome | Cushing syndrome (E240, E248, E249, E270) | Malignant neoplasm of adrenal gland medulla (C741) | Unilateral or bilateral adrenalectomy (P4571, P4572) | Pituitary gland surgery (S4633, S4743) | [84] |
| Ectopic Cushing syndrome (E243) or pituitary Cushing syndrome (E240) | |||||
| Congenital adrenal hyperplasia | ≥2 V115, E25/E25.0/E25.8/E25.9 (primary or secondary diagnosis) | Glucocorticoid or fludrocortisone for more than 6 months | [82] | ||
| Primary aldosteronism | ≥2 E26, I15.20, or I15.21 (primary or secondary diagnosis) | Secondary hyperaldosteronism (E26.1) | (Saline infusion test or the captopril challenge test) or adrenalectomy or prescription of spironolactone for >6 months | [83] | |
| Acromegaly | ≥2 Acromegaly (E22.0) | Acromegaly-related treatment (medical therapy, operation, or radiotherapy) within 2 years of the first medical claim for acromegaly | [85] | ||
| ≥1 Acromegaly (E22.0) or V112 | [86-88] | ||||
| Cushing disease | E24.0 and D35.2 | [90] | |||
| V162 and V114 | |||||
| Pituitary adenoma: V162 | |||||
| Cushing syndrome: V114 | |||||
| Prolactinoma | E22.1and D35.2 | [90] | |||
| V162 and V113 | |||||
| Pituitary adenoma: V162 | |||||
| Hyperprolactinemia: V113 | |||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download