1. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018; 138:271–81.

2. Lee KW, Ching SM, Ramachandran V, Yee A, Hoo FK, Chia YC, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018; 18:494.

3. Jung CH, Jung SH, Choi D, Kim BY, Kim CH, Mok JO. Gestational diabetes in Korea: temporal trends in prevalence, treatment, and short-term consequences from a national health insurance claims database between 2012 and 2016. Diabetes Res Clin Pract. 2021; 171:108586.

4. Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018; 19:3342.

5. HAPO Study Cooperative Research Group. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Int J Gynaecol Obstet. 2002; 78:69–77.
6. Hong S, Lee SM, Kwak SH, Kim BJ, Koo JN, Oh IH, et al. A comparison of predictive performances between old versus new criteria in a risk-based screening strategy for gestational diabetes mellitus. Diabetes Metab J. 2020; 44:726–36.

7. Kim MH, Kwak SH, Kim SH, Hong JS, Chung HR, Choi SH, et al. Pregnancy outcomes of women additionally diagnosed as gestational diabetes by the International Association of the Diabetes and Pregnancy Study Groups Criteria. Diabetes Metab J. 2019; 43:766–75.

8. Nombo AP, Mwanri AW, Brouwer-Brolsma EM, Ramaiya KL, Feskens EJM. Gestational diabetes mellitus risk score: a practical tool to predict gestational diabetes mellitus risk in Tanzania. Diabetes Res Clin Pract. 2018; 145:130–7.

9. Guo F, Yang S, Zhang Y, Yang X, Zhang C, Fan J. Nomogram for prediction of gestational diabetes mellitus in urban, Chinese, pregnant women. BMC Pregnancy Childbirth. 2020; 20:43.

10. Schaefer KK, Xiao W, Chen Q, He J, Lu J, Chan F, et al. Prediction of gestational diabetes mellitus in the Born in Guangzhou Cohort Study, China. Int J Gynaecol Obstet. 2018; 143:164–71.

11. Ramos-Levi AM, Perez-Ferre N, Fernandez MD, Del Valle L, Bordiu E, Bedia AR, et al. Risk factors for gestational diabetes mellitus in a large population of women living in Spain: implications for preventative strategies. Int J Endocrinol. 2012; 2012:312529.
12. Zheng T, Ye W, Wang X, Li X, Zhang J, Little J, et al. A simple model to predict risk of gestational diabetes mellitus from 8 to 20weeks of gestation in Chinese women. BMC Pregnancy Childbirth. 2019; 19:252.

13. Chi Z, Zhang S, Wang Y, Yang L, Yang Y, Li X. Research of gestational diabetes mellitus risk evaluation method. Technol Health Care. 2016; 24 Suppl 2:S499–503.

14. Kim HK, Song SO, Noh J, Jeong IK, Lee BW. Data configuration and publication trends for the Korean National Health Insurance and Health Insurance Review & Assessment Database. Diabetes Metab J. 2020; 44:671–8.

15. Kim MK, Han K, Lee SH. Current trends of big data research using the Korean National Health Information Database. Diabetes Metab J. 2022; 46:552–63.

16. Kim MK, Han K, You SY, Kwon HS, Yoon KH, Lee SH. Prepregnancy smoking and the risk of gestational diabetes requiring insulin therapy. Sci Rep. 2020; 10:13901.

17. Seo MH, Lee WY, Kim SS, Kang JH, Kang JH, Kim KK, et al. 2018 Korean Society for the Study of Obesity guideline for the management of obesity in Korea. J Obes Metab Syndr. 2019; 28:40–5.

18. Cho Y, Han K, Kim DH, Park YM, Yoon KH, Kim MK, et al. Cumulative exposure to metabolic syndrome components and the risk of dementia: a nationwide population-based study. Endocrinol Metab (Seoul). 2021; 36:424–35.

19. Lee SH, Han K, Kim HS, Cho JH, Yoon KH, Kim MK. Predicting the development of myocardial infarction in middleaged adults with type 2 diabetes: a risk model generated from a nationwide population-based cohort study in Korea. Endocrinol Metab (Seoul). 2020; 35:636–46.

20. Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, Sharma A. Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes. 2001; 50 Suppl 1:S154–9.

21. Kim MK, Han K, Koh ES, Hong OK, Baek KH, Song KH, et al. Cumulative exposure to impaired fasting glucose and future risk of type 2 diabetes mellitus. Diabetes Res Clin Pract. 2021; 175:108799.

22. Corrado F, D’Anna R, Cannata ML, Interdonato ML, Pintaudi B, Di Benedetto A. Correspondence between first-trimester fasting glycaemia, and oral glucose tolerance test in gestational diabetes diagnosis. Diabetes Metab. 2012; 38:458–61.

23. Riskin-Mashiah S, Younes G, Damti A, Auslender R. Firsttrimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care. 2009; 32:1639–43.

24. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010; 33:676–82.

25. Mills JL, Jovanovic L, Knopp R, Aarons J, Conley M, Park E, et al. Physiological reduction in fasting plasma glucose concentration in the first trimester of normal pregnancy: the diabetes in early pregnancy study. Metabolism. 1998; 47:1140–4.

26. Torloni MR, Betran AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009; 10:194–203.

27. Ogonowski J, Miazgowski T, Kuczynska M, KrzyzanowskaSwiniarska B, Celewicz Z. Pregravid body mass index as a predictor of gestational diabetes mellitus. Diabet Med. 2009; 26:334–8.

28. Kouhkan A, Khamseh ME, Moini A, Pirjani R, Arabipoor A, Zolfaghari Z, et al. Diagnostic accuracy of body mass index and fasting glucose for the prediction of gestational diabetes mellitus after assisted reproductive technology. Int J Fertil Steril. 2019; 13:32–7.
29. Kim WJ, Chung Y, Park J, Park JY, Han K, Park Y, et al. Influences of pregravid liver enzyme levels on the development of gestational diabetes mellitus. Liver Int. 2021; 41:743–53.

30. You SY, Han K, Lee SH, Kim MK. Nonalcoholic fatty liver disease and the risk of insulin-requiring gestational diabetes. Diabetol Metab Syndr. 2021; 13:90.

31. Zhang H, Forman HJ, Choi J. Gamma-glutamyl transpeptidase in glutathione biosynthesis. Methods Enzymol. 2005; 401:468–83.
32. Zhao W, Zhang L, Zhang G, Varkaneh HK, Rahmani J, Clark C, et al. The association of plasma levels of liver enzymes and risk of gestational diabetes mellitus: a systematic review and dose-response meta-analysis of observational studies. Acta Diabetol. 2020; 57:635–44.

33. Li Y, Wang X, Jiang F, Chen W, Li J, Chen X. Serum lipid levels in relation to clinical outcomes in pregnant women with gestational diabetes mellitus: an observational cohort study. Lipids Health Dis. 2021; 20:125.

34. Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG. 2015; 122:643–51.

35. Li Y, Ren X, He L, Li J, Zhang S, Chen W. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020; 162:108044.

36. Lean SC, Derricott H, Jones RL, Heazell AE. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. 2017; 12:e0186287.

37. Badon SE, Zhu Y, Sridhar SB, Xu F, Lee C, Ehrlich SF, et al. A pre-pregnancy biomarker risk score improves prediction of future gestational diabetes. J Endocr Soc. 2018; 2:1158–69.

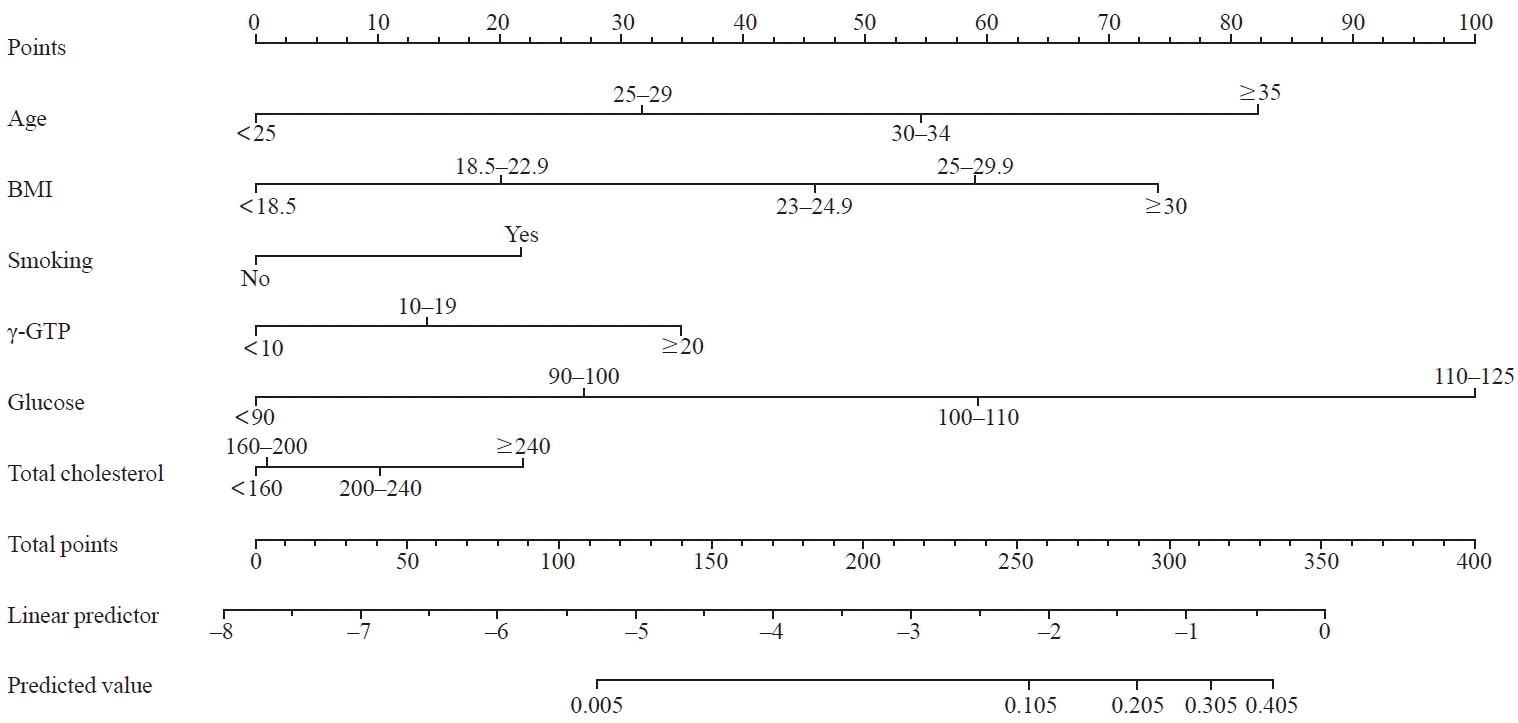
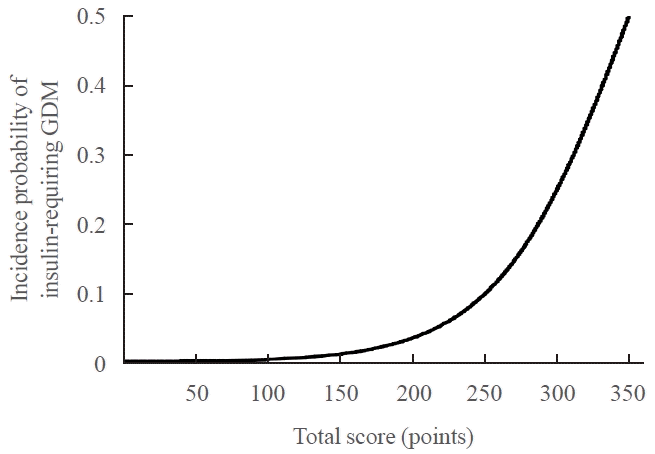
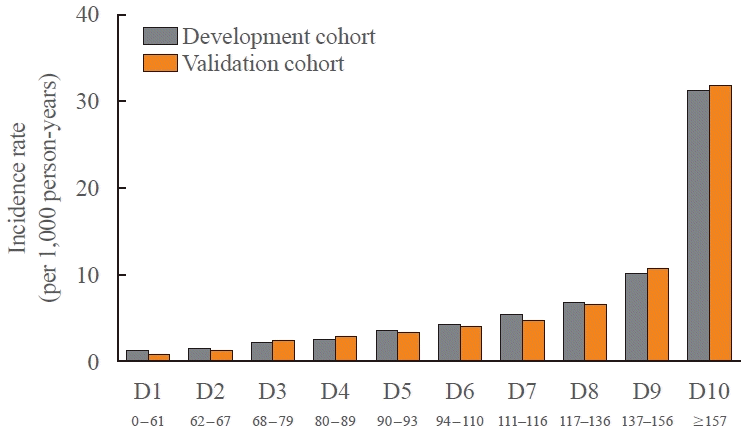




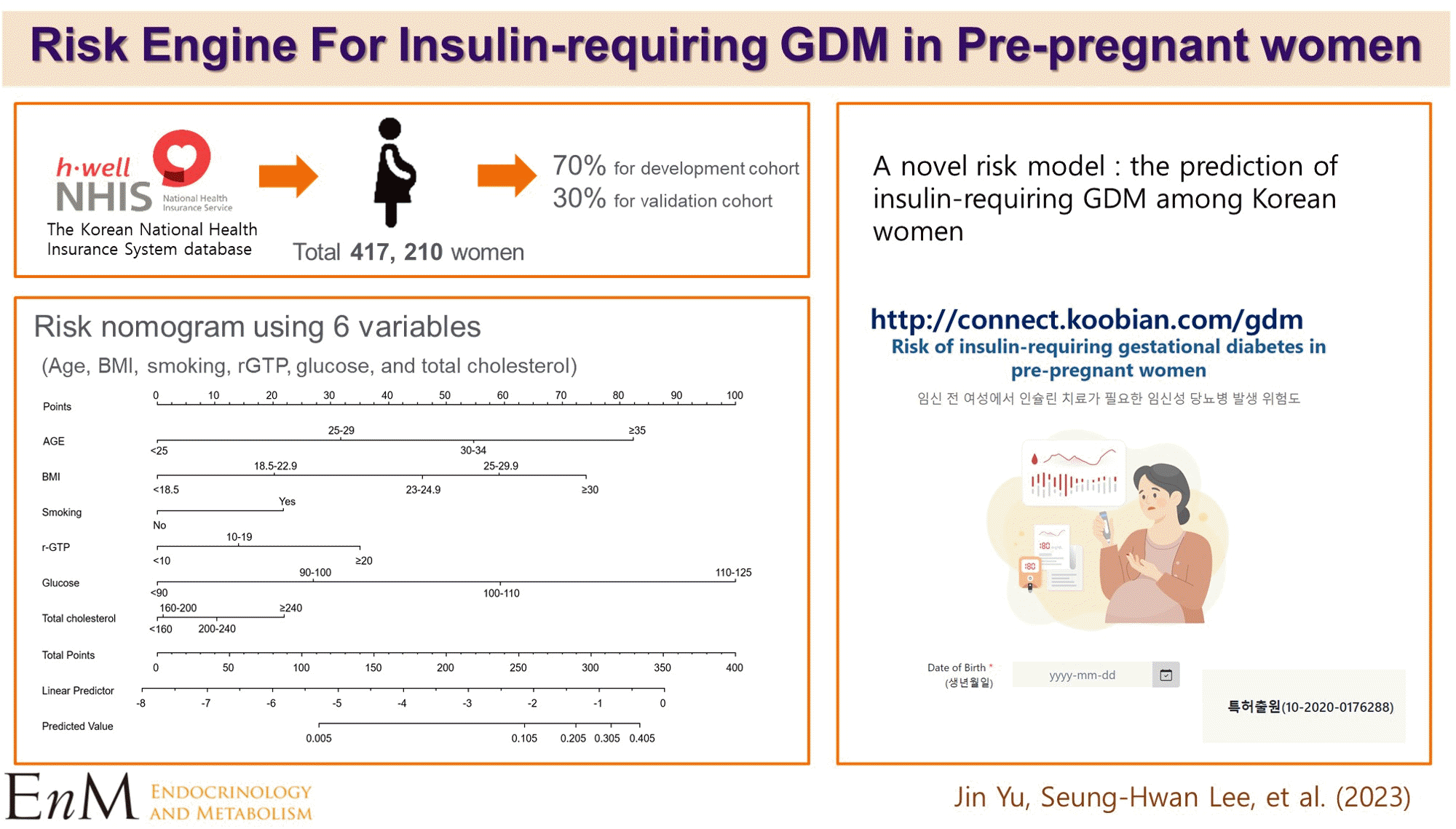
 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download