INTRODUCTION
METHODS
Literature search strategy
Inclusion and exclusion criteria
Data extraction
Quality assessment
Data synthesis and analysis
RESULTS
Literature search and eligibility criteria
Characteristics of the included studies
Table 1.
| Study | Country | Study design | Period | No. of included patients | Mean age (range), yr | No. of male patients (male proportion, %) | No. of nodules (≥1 cm) diagnosed with the reference standard | No. of malignant nodules (≥1 cm, malignancy rate, %) | Mean size (range), cm |
US-based RSSs |
Reference standard |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACR | ATA | EU | 2016 K | 2021 K | Surgery | Biopsy (FNA w/wo CNB) | US follow-upa | ||||||||||
| Chung et al. (2021) [33]b | Korea | Multicenter, retrospective | 06/2015–09/2015 | 5,081 | 53.2 (19–76) | 905 (17.8) | 5,708 | 1,111 (19.5) | 2.1 (1–10) | N | N | N | Y | Yc | Y | Y | N |
| Eidt et al. (2023) [32] | Brazil | Single, prospective | 01/2019–12/2021 | 149 | 55.0 (NA) | 20 (13.4) | 168 | 11 (6.5) | 2.6 (1.9–4.0)d | Y | N | Y | N | N | Y | Y | N |
| Grani et al. (2019) [16] | Italy | Single, prospective | 11/2015–05/2018 | 477 | 55.9 (NA) | 119 (24.9) | 502 | 36 (7.2) | NA | Y | Y | Y | Y | N | Y | Y | N |
| Ha et al. (2018) [20] | Korea | Multicenter, retrospective | 06/2013–05/2015 | 750 | 49.2 (9–81)e | 156 (20.8) | 586f | 101 (17.2)f | 1.5 (NA) | Y | Y | N | Y | N | Y | Y | N |
| Ha et al. (2018) [15] | Korea | Multicenter, retrospective | 01/2010–05/2011 | 1,802 | 51.2 (13–79) | 415 (23.0) | 2,000 | 454 (22.7) | 2 (1–NA) | Y | Y | N | Y | N | Y | Y | Y |
| Ha et al. (2021) [29]b | Korea | Multicenter, retrospective | 06/2015–09/2015 | 5,081 | 53.2 (19–93) | 905 (17.8) | 5,708 | 1,111 (19.5) | 2.1 (1–10) | Y | N | Y | N | Yg | Y | Y | N |
| Ha et al. (2019) [17] | Korea | Single, retrospective | 01/2013–12/2013 | 3,190 | 53.4 (14–94) | 673 (21.1) | 3,323 | 856 (25.8) | 1.4 (0.3–9.6) | Y | Y | N | Y | N | Y | Y | Y |
| Huh et al. (2021) [30] | Korea | Single, retrospective | 03/2017–01/2019 | 2,084 | 50.4 (19–92) | 433 (20.8) | 2,106 | 522 (24.8) | 2.3 (1–10) | Y | N | N | N | N | Y | Y | N |
| Middleton et al. (2018) [21] | USA | Multicenter, retrospective | 01/2006–12/2010 | 3,315 | 54.4 (18–97) | NA | 3,179h | 288 (9.1)f | NA | Y | Y | N | Y | N | Y | Y | N |
| Na et al. (2021) [31] | Korea | Single, retrospective | 01/2011–12/2019 | 3,088 | 56.0 (47–64)h | 591 (19.1) | 3,826 | 549 (14.3) | 1.7 (1–10)c | Y | Y | Y | Y | N | Y | Y | N |
| Tan et al. (2020) [28] | Malaysia | Single, retrospective | 08/2017–01/2020 | 128 | 51.8 (NA) | 21 (16.7) | 144 | 7 (4.9) | 2.1 (NA) | Y | N | Y | Y | N | N | Y | N |
RSS, risk stratification system; ACR, American College of Radiology; ATA, American Thyroid Association; EU, European; K, Korean; FNA, fine-needle aspiration; w/wo, with or without; CNB, core needle biopsy; US, ultrasonography; NA, not available.
a In studies that regarded follow-up as a reference standard, thyroid nodules with initial benign results on biopsy and decreased or stable size on follow-up US at more than 12 months were finally diagnosed as benign;
b These two studies shared the same study cohort with different purposes (2021 Korean Thyroid Imaging Reporting and Data System [K-TIRADS] vs. 2016 K-TIRADS [33]; 2021 K-TIRADS vs. foreign RSSs [29]). We representatively cited Chung et al. [33] for the outcomes from the 2021 K-TIRADS (2021 K-TIRADS1.0 or 2021 K-TIRADS1.5) throughout this study, unless it was necessary to specifically cite the study of Ha et al. [29];
c Modified K-TIRADS 1 and modified K-TIRADS 3 correspond to the 2021 K-TIRADS1.0 and 2021 K-TIRADS1.5, respectively;
f These numbers only represent those of thyroid nodules measuring ≥1 cm in each study, after excluding sub-centimeter nodules that were originally included in these study cohorts;
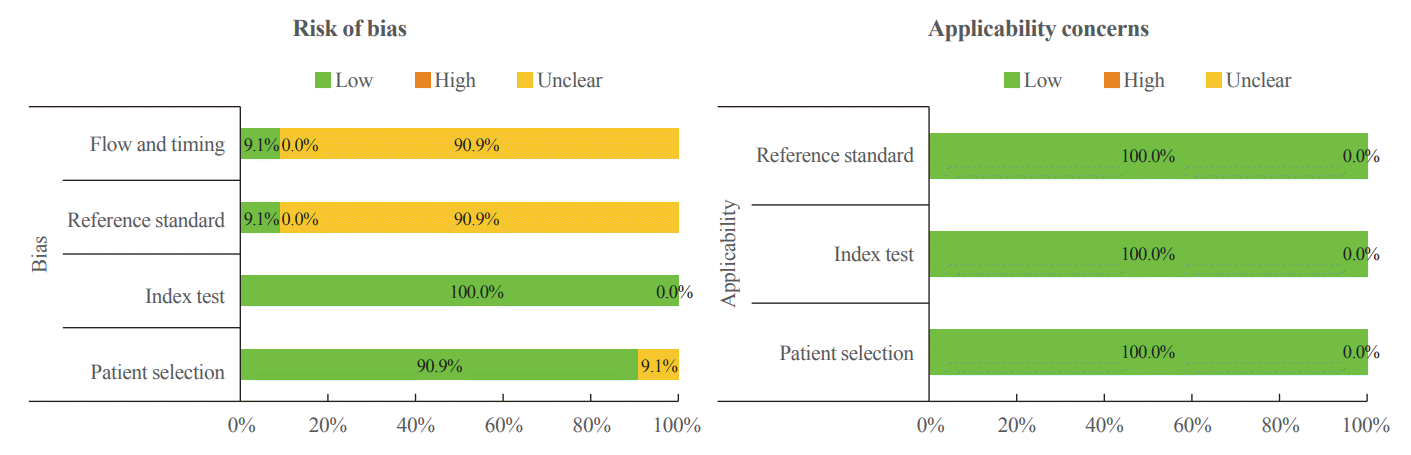
Quality assessment
Diagnostic performance
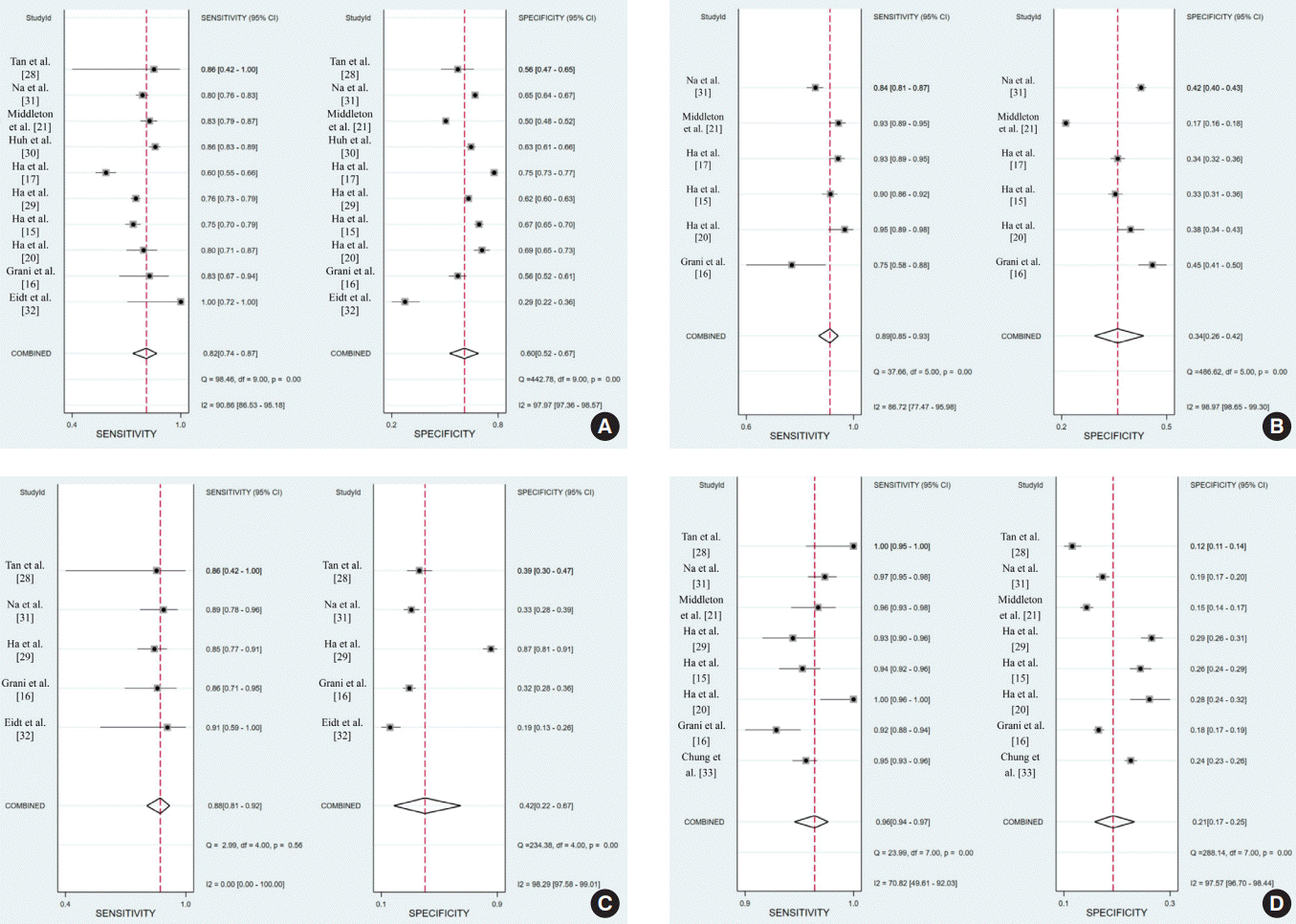 | Figure 3.Sensitivity and specificity of the (A) American College of Radiology (ACR)-Thyroid Imaging Reporting and Data System (TIRADS), (B) American Thyroid Association (ATA) system, (C) European (EU)-TIRADS, and (D) 2016 Korean (K)-TIRADS. CI, confidence interval. |
Table 2.
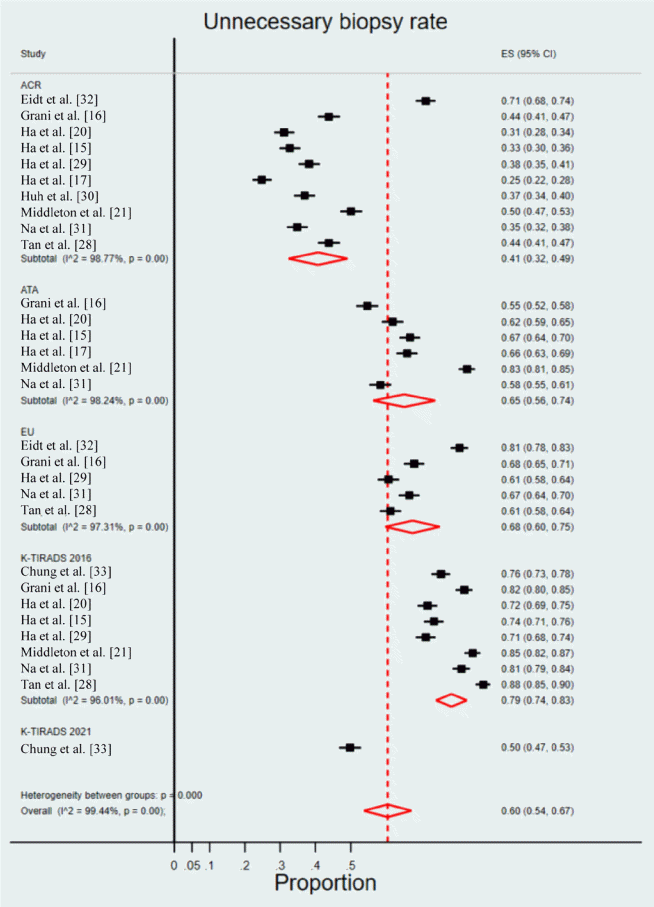
| RSS | Study | No. of included nodules (≥1 cm, malignant/total) | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | Unnecessary biopsy rate (1-specificity) |
|---|---|---|---|---|---|---|---|---|
| ACR-TIRADS | Eidt et al. (2023) [32] | 11/168 | 100 (100–100.0) | 28.7 (21.6–35.7) | 8.9 (3.9–14.0) | 100.0 (100–100.0) | 33.3 (26.3–41.0) | 71.3 |
| Grani et al. (2019) [16] | 36/502 | 83.3 (67.2–93.6) | 56.2 (51.6–60.8) | 12.8 (8.8–17.8) | 97.8 (95.2–99.2) | 58.2 (53.7–62.5) | 43.8 | |
| Ha et al. (2018) [20] | 101/586 | 80.2 (71.1–87.5) | 68.9 (64.5–73.0) | 34.9 (31.3–38.7) | 94.4 (91.8–96.1) | 70.8 (67.0–74.5) | 31.1 | |
| Ha et al. (2018) [15] | 454/2,000 | 74.7 (70.7–78.7) | 67.3 (65.0–69.7) | 40.2 (36.9–43.5) | 90.1 (88.3–91.8) | 69.0 (67.0–71.0) | 32.7 | |
| Ha et al. (2021) [29] | 1,111/5,708 | 76.1 (73.5–78.5) | 61.8 (60.4–63.2) | 32.5 (30.7–34.3) | 91.4 (90.4–92.4) | 64.6 (63.3–65.8) | 38.2 (36.8–39.6) | |
| Ha et al. (2019) [17] | 321/1,938 | 60.1 (54.5–65.5) | 75.2 (73.0–77.3) | 32.5 (29.9–35.3) | 90.5 (89.2–91.6) | 72.7 (70.7–74.7) | 24.8 | |
| Huh et al. (2021) [30] | 522/2,106 | 86.4 (83.5–89.3) | 63.1 (60.8–65.5) | 43.6 (40.6–46.6) | 93.4 (91.9–94.9) | 68.9 (66.9–70.9) | 36.9 | |
| Middleton et al. (2018) [21] | 288/3,179 | 83.3 (78.5–87.5) | 49.9 (48.1–52.8) | 14.2 (13.5–15.0) | 96.8 (95.9–97.5) | 53.0 (51.2–54.7) | 50.1 | |
| Na et al. (2021) [31] | 549/3,826 | 79.6 (76.0–82.9) | 65.2 (63.6–66.9) | 27.7 (25.5–30.0) | 95.0 (94.0–95.9) | 67.3 (65.8–68.8) | 34.8 | |
| Tan et al. (2020) [28] | 7/144 | 85.7 (42.7–97.4) | 56.2 (47.8–64.2) | 9.1 (6.5–12.5) | 98.7 (92.6–99.8) | 57.6 (49.1–65.8) | 43.8 | |
| ATA system | Grani et al. (2019) [16] | 36/502 | 75.0 (57.8–87.9) | 45.3 (40.7–49.9) | 9.6 (6.4–13.6) | 95.9 (92.4–98.1) | 47.4 (43.0–51.9) | 54.7 |
| Ha et al. (2018) [20] | 101/586 | 95.0 (88.8–98.4) | 38.1 (33.8–42.6) | 24.2 (22.8–25.8) | 97.4 (94.0–98.9) | 48.0 (43.8–52.1) | 61.9 | |
| Ha et al. (2018) [15] | 454/2,000 | 89.6 (86.9–92.5) | 33.2 (30.8–35.5) | 28.3 (25.9–30.6) | 91.6 (89.3–93.9) | 46.0 (43.8–48.2) | 66.8 | |
| Ha et al. (2019) [17] | 321/1,938 | 92.5 (89.1–95.2) | 34.0 (31.6–36.3) | 21.8 (21.0–22.6) | 95.8 (93.9–97.1) | 43.7 (41.4–45.9) | 66.0 | |
| Middleton et al. (2018) [21] | 288/3,179 | 92.7 (89.1–95.4) | 17.0 (15.7–18.4) | 10.0 (9.7–10.4) | 95.9 (93.9–97.3) | 23.9 (22.4–25.4) | 83.0 | |
| Na et al. (2021) [31] | 549/3,826 | 84.0 (80.6–86.9) | 41.6 (39.9–43.3) | 19.4 (17.8–21.1) | 93.9 (92.6–95.1) | 47.7 (46.0–49.2) | 58.4 | |
| EU-TIRADS | Eidt et al. (2023) [32] | 11/168 | 90.9 (73.9–99.9) | 19.1 (13.0–25.3) | 7.3 (2.9–11.7) | 96.8 (90.6–100.0) | 23.8 (17.6–31.0) | 80.9 |
| Grani et al. (2019) [16] | 36/502 | 86.1 (70.5–95.3) | 32.0 (27.8–36.4) | 8.9 (6.1–12.4) | 96.7 (92.6–98.9) | 35.9 (31.7–40.2) | 68.0 | |
| Ha et al. (2021) [29] | 1,111/5,708 | 84.6 (82.4–86.6) | 39.3 (37.9–40.7) | 25.2 (23.8–26.6) | 91.4 (90.0–92.5) | 48.1 (46.8–49.4) | 60.7 (59.3–62.1) | |
| Na et al. (2021) [31] | 549/3,826 | 88.3 (85.4–90.9) | 33.4 (31.7–35.0) | 18.2 (16.7–19.7) | 94.5 (93.0–95.7) | 41.2 (39.7–42.8) | 66.6 | |
| Tan et al. (2020) [28]a | 7/144 | 85.7 (42.1–99.6) | 38.7 (30.5–47.4) | 6.7 (4.9–9.0) | 98.2 (89.5–99.7) | 41.0 (32.9–49.5) | 61.3 | |
| 2016 K-TIRADS | Chung et al. (2021) [33] | 1,111/5,708 | 94.9 (93.4−96.0) | 24.4 (23.2−25.7) | 23.3 (22.1−24.5) | 95.2 (93.8−96.3) | 38.1 (36.9−39.4) | 75.6 |
| Grani et al. (2019) [16] | 36/502 | 91.7 (77.5–98.2) | 17.8 (14.4–21.6) | 7.9 (5.5–11) | 96.5 (90.2–99.3) | 23.1 (19.5–27.1) | 82.2 | |
| Ha et al. (2018) [20] | 101/586 | 100 (96.4–100) | 28.2 (24.3–32.5) | 22.5 (21.5–23.5) | 100 | 40.6 (36.6–44.7) | 71.8 | |
| Ha et al. (2018) [15] | 454/2,000 | 94.5 (92.4, 96.6) | 26.4 (24.2–28.6) | 27.4 (25.2–29.6) | 94.2 (92.0–96.4) | 41.9 (39.7–44.0) | 73.6 | |
| Ha et al. (2021) [29] | 321/1,938 | 93.5 (90.2–95.9) | 28.7 (26.5–31.0) | 20.6 (20.0–21.4) | 95.7 (93.6–97.1) | 39.4 (37.2–41.6) | 71.3 | |
| Middleton et al. (2018) [21] | 288/3,179 | 96.2 (93.3–98.1) | 15.4 (14.1–16.7) | 10.2 (9.9–10.4) | 97.6 (95.7–98.6) | 22.7 (21.2–24.2) | 84.6 | |
| Na et al. (2021) [31] | 549/3,826 | 96.9 (95.1–98.2) | 18.6 (17.3–20.0) | 16.6 (15.4–18.0) | 97.3 (95.7–98.4) | 29.9 (28.4–31.4) | 81.4 | |
| Tan et al. (2020) [28] | 7/144 | 100 (59.0–100) | 12.4 (7.4–19.1) | 5.5 (5.2–5.9) | 100 (100–100.0) | 16.7 (11.0–23.8) | 87.6 | |
| 2021 K-TIRADS1.0a | Chung et al. (2021) [33] | 1,111/5,708 | 91.0 (89.2−92.5) | 39.7 (38.3−41.1) | 26.7 (25.3−28.2) | 94.8 (93.7−95.7) | 49.7 (48.4−51.0) | 60.3 (58.9−61.7) |
| 2021 K-TIRADS1.5b | Chung et al. (2021) [33] | 1,111/5,708 | 76.1 (73.6−78.6) | 50.2 (48.7−51.6) | 27.0 (25.5−28.6) | 89.7 (88.5−90.8) | 55.2 (53.9−56.5) | 49.8 (48.4–51.3) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download