Abstract
Background
There have concerns related with the potential harms of fine-needle aspiration biopsy (FNAB). We aimed to summarize the clinical complications and evaluate the safety of FNAB.
Methods
Studies related with the harms of FNAB were searched on MEDLINE, Embase, Cochrane library, and KoreaMed from 2012 to 2022. Also, studies reviewed in the previous systematic reviews were evaluated. Included clinical complications were postprocedural pain, bleeding events, neurological symptoms, tracheal puncture, infections, post-FNAB thyrotoxicosis, and needle tract implantation of thyroid cancers.
Results
Twenty-three cohort studies were included in this review. Nine studies which were related with FNAB-related pain showed that most of the subjects had no or mild discomfort. The 0% to 6.4% of the patients had hematoma or hemorrhage after FNAB, according to 15 studies. Vasovagal reaction, vocal cord palsy, and tracheal puncture have rarely described in the included studies. Needle tract implantation of thyroid malignancies was described in three studies reporting 0.02% to 0.19% of the incidence rate.
Fine-needle aspiration biopsy (FNAB) is the most wildly accepted preoperative diagnostic technique for thyroid malignancies because of its high accuracy, safety, simplicity, and cost-effectiveness [1,2]. The cytological findings obtained from FNAB are used to classify thyroid nodules into various categories in thyroid imaging reporting and data systems, and to decide whether to perform surgery or conduct surveillance [3-5]. FNAB can be performed with guidance by palpation or ultrasound, the latter of which has become more popular in recent years, and the specimen can be obtained by a needle attached to a syringe or by using capillary action [6]. Although FNAB is considered to be very safe, it is important to be aware of the clinical complications that can occur after FNAB, as the increasing number of performed FNAB procedures can lead to an increased frequency of clinical complications. Herein, we present a thorough review of FNAB-related adverse events to evaluate the safety of FNAB and help clinicians to have a comprehensive perspective on the procedure to prevent complications.
We performed a literature search on MEDLINE, Embase, Cochrane Library, and KoreaMed from 2012 to 2022. We also reviewed references from previous systematic reviews, reviews and consensus statements related to the complications of FNAB [7-9]. The search strategy was conducted using medical terms (“thyroid neoplasm,” “ultrasonography,” “fine needle aspiration biopsy,” “complication”). The study protocol with detailed search strategies is included in Appendix 1. We reported the results in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Supplemental Table S1). Only articles written in English were included.
First, two reviewers (J.Y.P. and H.K.K.) independently reviewed the titles and abstracts, and excluded irrelevant articles. Any discrepancies between the two reviewers were settled by consultation with a third investigator (H.C.K.). A full-text review was performed following the inclusion criteria as follows: (1) studies designed as cohort studies; (2) adult patients who underwent ultrasound-guided FNAB for thyroid nodules; (3) studies reporting clinical complication rates related to FNAB; and (4) studies that were written in English. The following studies were excluded: (1) studies including patients under 18 years of age; (2) studies with patients who did not undergo FNAB; and (3) studies that did not mention the adverse events of FNAB.
Two independent reviewers (J.Y.P. and H.K.K.) extracted the data as follows: first author’s name, country where the study was done, publication year, number of cases and patients who underwent FNAB, study design, age, sex, gauge size of the needles used for FNAB, and outcomes related to clinical harms after FNAB.
Quality assessment was done using the Cochrane Risk of Bias tool 2.0 for randomized controlled studies and the Newcastle-Ottawa Quality Assessment Scale for non-randomized studies (Supplemental Table S2, Supplemental Fig. S1). After a thorough assessment using items of the Newcastle-Ottawa Quality Assessment Scale (selection of studies, comparability, and outcomes), we rated the scores of the studies. Any disagreements were resolved by a third reviewer (H.C.K.).
Ethical approval can be waived as this study was not performed on human participants.
The study protocol is registered in International Prospective Register of Systematic Reviews (PROSPERO) with the title of: “Comprehensive assessment of harms caused by fine-needle aspiration for screening and diagnosing thyroid neoplasms: a systematic review (ID: CRD42022364961).”
The flow diagram of the present study is described in Fig. 1. A total of 199 studies were initially identified, and 59 remained after the first screening. The excluded studies included reviews, case reports or case series, studies with different topics of interest, meta-analyses, and conference abstracts. After reviewing 59 studies based on the full-text, 16 studies were finally included for review. Moreover, seven studies were added through an additional literature search, resulting in 23 studies in total. The characteristics of the studies relevant to the clinical harms of ultrasound-guided FNAB are summarized in Table 1. Three randomized studies and 20 non-randomized studies were analyzed.
According to the nine studies that reported pain at the puncture site during FNAB, most of the patients showed mild or no pain, although the pain rating scales were inconsistent [10-18]. Researchers tried to find any significant factors that would alleviate pain during the procedure. A comparative study conducted by Lee et al. [12] showed similar results of pain scales according to whether patients underwent FNAB with 23-gauge needles or 25-gauge needles. Jung et al. [13] also reported consistent results that the mean pain scale values in the 21-gauge needle group and the 23-gauge needle group were 1.8±1.3 (range, 0 to 6) and 1.4±1.1 (range, 0 to 5), respectively, showing no statistically significant difference depending on the gauge size. The association between the characteristics of thyroid nodules and the severity of pain was also evaluated. Cordes et al. [14] noted that neither the volume nor the type of thyroid nodules showed any significant differences in terms of adverse event rates, mainly pain at the puncture site. Toman et al. [15] emphasized that the depth of thyroid nodules was correlated with the pain score, and recommended using anesthesia to alleviate FNAB-induced pain for patients who had thyroid nodules in the deep portion of their necks. As some patients are very sensitive to pain, the efficacy of anesthesia when performing FNAB has been an issue. Gursoy et al. [17,18] reported in their randomized double-blind trial that local anesthesia prominently alleviated pain after FNAB. The relationship between the expertise of the clinicians and pain after FNAB was also evaluated. A comparative study by Lee et al. [16] analyzed the pain scores of two groups divided according to the career of the operator (≥1,200 ultrasound-guided FNAB procedures per year for 10 years vs. 500 ultrasound-guided FNAB procedures for 1 year) and did not show any statistically significant difference.
Fifteen studies describing episodes of bleeding events or hematoma are listed in Table 1 [11,13,14,16,19-29]. Some studies showed superior or non-inferior safety outcomes of FNAB compared to those of core needle biopsy (CNB). Ahn et al. [22] reported that no complications took place in the FNAB group, while perithyroidal or intrathyroidal hemorrhage occurred in 0.7% (5/705) of patients in the CNB group, without a significant difference (P=0.069). Kim et al. [23] stated that the incidence of subcapsular hematoma was similar in the FNAB group (3/87) and the CNB group (2/80). Chae et al. [24] reported that 0.8% (43/5,121) of the patients in FNAB group and 4.9% (9/183) of the patients in CNB group had hematoma, and emphasized that CNB was the only significant factor associated with an increased risk of post-biopsy bleeding events, showing an adjusted odds ratio of 6.458 (95% confidence interval, 2.348 to 17.766; P<0.001). Khoo et al. [25] stated that three patients in the FNAB-only group (n=311) and eight patients in the FNAB and CNB group (n=320) had bleeding events. Chen et al. [26] reported one episode of minor hematoma in both the FNAB (n=96) and CNB (n=365) groups, respectively.
The discontinuation of anticoagulants or antithrombotic agents has also been an issue for clinicians when performing ultrasound-guided fine-needle aspiration. Khadra et al. [28] stated that the incidence of hematoma was similar between the antithrombotic/anticoagulant (AT/AC) group and the control group. Abu-Yousef et al. [29] also emphasized that the risk of bleeding events was not significantly different between patients taking AT/AC medications and patients not using AT/AC medications. Cordes et al. [14] reported two cases of minor hematomas, but the odds ratio for patients taking anticoagulant medications versus patients not taking anticoagulants did not show any statistical significance.
Five studies included in this study reported neurological symptoms after FNAB [14,19-21,30]. Cordes et al. [14] stated that 3.9% of patients experienced paresthesia and 0.5% of patients had dysphonia episodes after FNAB. Kavanagh et al. [19] and Cappelli et al. [21] reported incidence rates of 0.3% and 0.02% of vasovagal reaction after performing FNAB, respectively. Tomoda et al. [30] reported that four patients had transient vocal cord paralysis after reviewing 10,974 patients retrospectively. Newkirk et al. [20] found in their retrospective study that transient hoarseness occurred in 0.9% and vasovagal reaction in 1.3% of patients who underwent FNAB.
Of the included studies, one study described episodes of tracheal puncture. Cappelli et al. [21] reported that tracheal puncture occurred in 0.04% of enrolled patients (2/6,323) during FNAB.
Several studies reported the risk of needle tract implantation (NTI) of thyroid cancer after FNAB. Ito et al. [31] reported that NTI occurred in 0.14% (7/4,912) of papillary thyroid cancer patients and that the age of the patients and aggressive characteristics of the tumor were risk factors for NTI. They also reported a case of subcutaneous seeding of follicular neoplasm after FNAB [32]. However, based on their results, Ito et al. [31,32] noted that the incidence of NTI was very rare and that NTI had high cure rates after surgery; therefore, they concluded that FNAB should not be discouraged for the preoperative diagnosis of thyroid malignancies. Cappelli et al. [21] also reported a very low incidence of cancer implantation along the needle tract (0.02%, 1/6,323). A large study conducted by Hayashi et al. [33] stated that 0.19% of the patients (22/11,745) experienced NTI of thyroid cancer.
FNAB is a safe, simple, cost-effective, and highly accurate diagnostic modality for thyroid malignancies and has long been the gold standard. FNAB-related complications are rare, but it is crucial to be aware of various types of adverse events so that we can predict and prepare for potential complications.
Associations between pain after FNAB, which is the most common complication, and several clinical factors have been evaluated. Various sizes of needles ranging from 21 to 27 gauge have been used in clinical practice, and it is believed that larger needles might cause more pain without a difference in cytologic adequacy [13,21,34]. Although it was not statistically significant, multiple studies have shown lower pain scale scores in groups using smaller needles, which has prompted operators to favor smaller needles [12,13]. The depth of nodules, but not their volume, has been found to be related to the severity of pain during FNAB [14,15]. FNAB is generally performed without local anesthesia and the patients usually do not need any previous preparation, but some patients who are very sensitive to pain or are anxious about needles may require anesthesia before doing the procedure [6]. It is noteworthy that the efficacy of local anesthesia for FNAB was proven by Gursoy et al. [17,18]. It seems reasonable to use local anesthesia to relieve pain when clinicians perform FNAB for thyroid nodules located in the deep portion of the patient’s neck, as well as in patients who are intolerant to pain or anxious [15,17,18]. It is well known that the examiner’s expertise is important to obtain excellent diagnostic performance [6]. An interesting study conducted by Lee et al. [16] showed similar outcomes in terms of cytological adequacy and complications, including pain scale ratings between two operators with different expertise; the researchers concluded that a clinician with experience of over 500 ultrasound-guided FNAB procedures can be expected to have comparable performance to a more experienced clinician.
Hematoma is also a rare, but non-negligible complication after FNAB. According to recent comparative studies, FNAB consistently showed comparable or better outcomes related to bleeding events than FNAB [22-26]. Stopping anticoagulants or antithrombotic agents before performing FNAB has been debated. Based on recent studies showing no statistical significance between patient groups with or without anticoagulants or antithrombotic agents, researchers concluded that clinicians should decide whether to discontinue anticoagulants or antithrombotic agents based on patients’ individual circumstances due to the low risk of bleeding events after FNAB of thyroid nodules [9,14,28,29]. Unfortunately, rare but sometimes fatal thyroid hemorrhages after FNAB requiring intubation or surgery have been reported in case reports. Bonsignore et al. [35] reported a case of a 78-year-old woman who died due to a massive hematoma leading to tracheal compression and conducted a literature review of 12 cases of fatal thyroid hemorrhages, which were mostly managed with surgery or conservative measures [35-43]. Based on the fact that both cases of death identified in the literature review were in older women, the authors stated that increased venous fragility with age and arteriovenous shunts might have contributed to a higher risk of fatal vascular complications [35,44,45]. It would be advisable to thoroughly investigate the bleeding risk of patients before FNAB, although routine screening for anticoagulants or antithrombotic agents is not mandatory, and Doppler ultrasonography of the thyroid nodule should be performed before the examination, especially for hypervascular nodules and older patients [9].
Needle tract seeding with FNAB can occur by disseminated cancer cells when withdrawing the needle during FNAB. Reports stated that the incidence of NTI was very low, which could be explained by the indolent characteristics of papillary thyroid cancers [21,31-33]. Hayashi et al. [33] reported that NTI had a higher incidence from lymph nodes than from thyroid nodules and more commonly occurred from follicular thyroid cancers and anaplastic thyroid cancers than from papillary thyroid cancers, showing the importance of tumor aggressiveness for the occurrence and potential histopathological transformation of NTI. Most NTI cases could be controlled with surgical resection, but we should still be cautious when performing FNAB because NTI could possibly affect the patient’s disease course [31,33].
Episodes of voice change were reported in a few studies [14,20,30]. Possible mechanisms of recurrent laryngeal nerve palsy after FNAB include rapid stretching of the nerve caused by thyroid swelling or direct needle injury to the nerve, which can be prevented by not stabbing the dorsal part of the nodule and evaluating the vascularity of the thyroid nodule before performing FNAB [30]. Exceedingly rare complications that were reported in limited studies include vasovagal reactions after FNAB and tracheal puncture [19-21].
Because of its various protective mechanisms against pathogens, the incidence of infection within the thyroid gland after FNAB is very rare. Park and Jeon [46] reported a case of a 33-year-old immunocompetent woman who suffered from acute suppurative thyroiditis after FNAB and was successfully treated with antibiotics. Moreover, based on their literature review of five cases of FNAB-related thyroid infections, in which most patients had impaired immunity, they emphasized that clinicians should be aware of potential infections after FNAB, particularly in immunocompromised patients [46-51].
Post-aspiration thyrotoxicosis has been described in a limited number of reports [49,52,53]. The researchers stated that most of the patients had large nodules, and suggested that destructive thyroiditis caused by the traumatic leakage of thyroid hormones might cause thyrotoxicosis after FNAB [52,53]. We summarized the key points for preventing FNAB-related complications in Table 2.
The present study has several limitations. First, most of the included studies had small sample sizes and retrospective designs, which might weaken the conclusions of the present study. Second, the measures of the outcomes of interest were not unified (e.g., different pain scales or inconsistent outcomes of interest among the studies), which made it difficult to analyze the summarized data in greater detail. Moreover, as pain rating scales have subjective characteristics, some degree of bias would be inevitable. Third, the gauge of the needles was not unified, although needle size can affect some of the outcomes of interest.
In conclusion, FNAB of thyroid nodules is a safe diagnostic technique with a very low complication rate. We believe that the results related to adverse events from recent studies are insufficient to discourage performing FNAB for preoperative diagnoses of thyroid malignancies. Performing FNAB with special caution based on individual circumstances is recommended to prevent possible complications.
Supplementary Material
Supplementary Table S2.
Newcastle-Ottawa Quality Assessment Scale for Non-Randomized Studies
Supplementary Fig. S1.
Quality assessment of included randomized controlled trials according to the Risk of Bias tool 2.0 (RoB 2.0).
ACKNOWLEDGMENTS
We acknowledge and thank Miyoung Choi (National Evidence-based Healthcare Collaborating Agency, Division of Health Technology Assessment Research) and Chang Hee Cho (The Korean Society of Radiology), who contributed to searching and interpreting evidence. This study was supported by the Korean Thyroid Association and research funding from the National Cancer Center (grant number 2112570-3).
REFERENCES
1. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.

2. Rumack CM, Wilson SR, Charboneau JW. Charboneau JW. Diagnostic ultrasound. Vol. 1. 3rd ed. Philadelphia: Elsevier Mosby;2005. p. 736–43.
3. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017; 6:225–37.

4. Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, et al. 2021 Korean Thyroid Imaging Reporting and Data System and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2021; 22:2094–123.

5. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017; 14:587–95.

6. Polyzos SA, Kita M, Avramidis A. Thyroid nodules: stepwise diagnosis and management. Hormones (Athens). 2007; 6:101–19.
7. Polyzos SA, Anastasilakis AD. Clinical complications following thyroid fine-needle biopsy: a systematic review. Clin Endocrinol (Oxf). 2009; 71:157–65.

8. US Preventive Services Task Force. Screening for thyroid cancer: US preventive services task force recommendation statement. JAMA. 2017; 317:1882–7.
9. Lee YH, Baek JH, Jung SL, Kwak JY, Kim JH, Shin JH. Ultrasound-guided fine needle aspiration of thyroid nodules: a consensus statement by the Korean Society of Thyroid Radiology. Korean J Radiol. 2015; 16:391–401.

10. Kim DW, Choo HJ, Park JS, Lee EJ, Kim SH, Jung SJ, et al. Ultrasonography-guided fine-needle aspiration cytology for thyroid nodules: an emphasis on one-sampling and biopsy techniques. Diagn Cytopathol. 2012; 40 Suppl 1:E48–54.

11. Birgi E, Ergun O, Turkmenoglu TT, Gunes Tatar I, Durmaz HA, Hekimoglu B. The contribution of vacuum-assisted modified Menghini type needle to diagnosis of US-guided fine needle aspiration biopsy of the thyroid. Diagn Interv Radiol. 2016; 22:173–7.

12. Lee YJ, Kim DW, Shin GW, Heo YJ, Baek JW, Choo HJ, et al. Comparison of cytological adequacy and pain scale score in ultrasound-guided fine-needle aspiration of solid thyroid nodules for liquid-based cytology with 23- and 25-gauge needles: a single-center prospective study. Sci Rep. 2019; 9:7027.
13. Jung SJ, Kim DW, Baek HJ. Comparison study of the adequacy and pain scale of ultrasound-guided fine-needle aspiration of solid thyroid nodules with a 21- or 23-gauge needle for liquid-based cytology: a single-center study. Endocr Pathol. 2018; 29:30–4.

14. Cordes M, Schmidkonz C, Horstrup K, Weppler M, Kuwert T. Fine-needle aspiration biopsies of thyroid nodules. Nuklearmedizin. 2018; 57:211–5.

15. Toman H, Ozkul F, Erbag G, Erbas M, Simsek T, Adam G, et al. Effects of fine-needle aspiration biopsy (FNAB) nodule depth on pain score. Ir J Med Sci. 2016; 185:673–6.

16. Lee YJ, Kim DW, Jung SJ. Comparison of sample adequacy, pain-scale ratings, and complications associated with ultrasound-guided fine-needle aspiration of thyroid nodules between two radiologists with different levels of experience. Endocrine. 2013; 44:696–701.

17. Gursoy A, Ertugrul DT, Sahin M, Tutuncu NB, Demirer AN, Demirag NG. Needle-free delivery of lidocaine for reducing the pain associated with the fine-needle aspiration biopsy of thyroid nodules: time-saving and efficacious procedure. Thyroid. 2007; 17:317–21.

18. Gursoy A, Ertugrul DT, Sahin M, Tutuncu NB, Demirer AN, Demirag NG. The analgesic efficacy of lidocaine/prilocaine (EMLA) cream during fine-needle aspiration biopsy of thyroid nodules. Clin Endocrinol (Oxf). 2007; 66:691–4.

19. Kavanagh J, McVeigh N, McCarthy E, Bennett K, Beddy P. Ultrasound-guided fine needle aspiration of thyroid nodules: factors affecting diagnostic outcomes and confounding variables. Acta Radiol. 2017; 58:301–6.

20. Newkirk KA, Ringel MD, Jelinek J, Mark A, Wartofsky L, Deeb ZE, et al. Ultrasound-guided fine-needle aspiration and thyroid disease. Otolaryngol Head Neck Surg. 2000; 123:700–5.

21. Cappelli C, Pirola I, Agosti B, Tironi A, Gandossi E, Incardona P, et al. Complications after fine-needle aspiration cytology: a retrospective study of 7449 consecutive thyroid nodules. Br J Oral Maxillofac Surg. 2017; 55:266–9.

22. Ahn HS, Youn I, Na DG, Kim SJ, Lee MY. Diagnostic performance of core needle biopsy as a first-line diagnostic tool for thyroid nodules according to ultrasound patterns: comparison with fine needle aspiration using propensity score matching analysis. Clin Endocrinol (Oxf). 2021; 94:494–503.

23. Kim HJ, Kim YK, Moon JH, Choi JY, Choi SI. Thyroid core needle biopsy: patients’ pain and satisfaction compared to fine needle aspiration. Endocrine. 2019; 65:365–70.

24. Chae IH, Kim EK, Moon HJ, Yoon JH, Park VY, Kwak JY. Ultrasound-guided fine needle aspiration versus core needle biopsy: comparison of post-biopsy hematoma rates and risk factors. Endocrine. 2017; 57:108–14.

25. Khoo TK, Baker CH, Hallanger-Johnson J, Tom AM, Grant CS, Reading CC, et al. Comparison of ultrasound-guided fine-needle aspiration biopsy with core-needle biopsy in the evaluation of thyroid nodules. Endocr Pract. 2008; 14:426–31.

26. Chen BT, Jain AB, Dagis A, Chu P, Vora L, Maghami E, et al. Comparison of the efficacy and safety of ultrasound-guided core needle biopsy versus fine-needle aspiration for evaluating thyroid nodules. Endocr Pract. 2015; 21:128–35.

27. Uchida T, Himuro M, Komiya K, Goto H, Takeno K, Honda A, et al. Evanescent hyperechoic changes after fine-needle aspiration biopsy of the thyroid in a series with a low overall prevalence of complications. J Ultrasound Med. 2016; 35:599–604.

28. Khadra H, Kholmatov R, Monlezun D, Kandil E. Do anticoagulation medications increase the risk of haematoma in ultrasound-guided fine needle aspiration of thyroid lesions? Cytopathology. 2018; 29:565–8.

29. Abu-Yousef MM, Larson JH, Kuehn DM, Wu AS, Laroia AT. Safety of ultrasound-guided fine needle aspiration biopsy of neck lesions in patients taking antithrombotic/anticoagulant medications. Ultrasound Q. 2011; 27:157–9.

30. Tomoda C, Takamura Y, Ito Y, Miya A, Miyauchi A. Transient vocal cord paralysis after fine-needle aspiration biopsy of thyroid tumor. Thyroid. 2006; 16:697–9.

31. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Needle tract implantation of papillary thyroid carcinoma after fine-needle aspiration biopsy. World J Surg. 2005; 29:1544–9.

32. Ito Y, Asahi S, Matsuzuka F, Nakamura Y, Amino N, Miyauchi A. Needle tract implantation of follicular neoplasm after fine-needle aspiration biopsy: report of a case. Thyroid. 2006; 16:1059–62.

33. Hayashi T, Hirokawa M, Higuchi M, Kudo T, Ito Y, Miyauchi A. Needle tract implantation following fine-needle aspiration of thyroid cancer. World J Surg. 2020; 44:378–84.

34. Tangpricha V, Chen BJ, Swan NC, Sweeney AT, de las Morenas A, Safer JD. Twenty-one-gauge needles provide more cellular samples than twenty-five-gauge needles in fine-needle aspiration biopsy of the thyroid but may not provide increased diagnostic accuracy. Thyroid. 2001; 11:973–6.

35. Bonsignore A, Drommi M, Frigiolini F, Roncallo A, Ventura F, Buffelli F, et al. A rare case of fatal thyroid hemorrhage after fine-needle aspiration: case report and review of the literature. Am J Forensic Med Pathol. 2022; 43:291–5.

36. Katagiri H, Lefor AK, Kubota T, Mizokami K, Kishida A. Massive hematoma after fine-needle aspiration of the thyroid. Surgery. 2016; 160:245–6.

37. Noordzij JP, Goto MM. Airway compromise caused by hematoma after thyroid fine-needle aspiration. Am J Otolaryngol. 2005; 26:398–9.

38. Yoshida M, Miyata M, Maeda H, Oiso Y. Massive thyroid hematoma developing after a fine-needle aspiration biopsy. Intern Med. 2012; 51:3223–4.

39. Donatini G, Masoni T. Is fine-needle aspiration cytology for thyroid nodules a routine and safe procedure? A series of emergency cervicotomies following FNAC. Langenbecks Arch Surg. 2010; 395:873–6.

40. Park MH, Yoon JH. Anterior neck hematoma causing airway compression following fine needle aspiration cytology of the thyroid nodule: a case report. Acta Cytol. 2009; 53:86–8.
41. Lee YS, Baek HS, Park TS, Jin HY. Bilateral intrathyroidal hemorrhage after fine needle aspiration completely resolved by compression without thyroidectomy. Endocrine. 2013; 43:460–1.

42. Roh JL. Intrathyroid hemorrhage and acute upper airway obstruction after fine needle aspiration of the thyroid gland. Laryngoscope. 2006; 116:154–6.

43. Hor T, Lahiri SW. Bilateral thyroid hematomas after fine-needle aspiration causing acute airway obstruction. Thyroid. 2008; 18:567–9.

44. Kakiuchi Y, Idota N, Nakamura M, Ikegaya H. A fatal case of cervical hemorrhage after fine needle aspiration and core needle biopsy of the thyroid gland. Am J Forensic Med Pathol. 2015; 36:207–9.

45. Strachan MW, Dalvi M, Ainsworth R, Gibb FW, Horsfall H, Patel D. Fatal haemorrhage following fine needle aspiration of the thyroid. J R Coll Physicians Edinb. 2016; 46:166–7.

46. Park JK, Jeon EJ. Infection of thyroid cyst occurring 1 month after fine-needle aspiration in an immunocompetent patient. Int J Thyroidol. 2018; 11:182–8.

47. Wang YC, Yeh TS, Lin JD. Gram-negative thyroid abscess resulting from fine-needle aspiration in an immunosuppressed patient. Clin Infect Dis. 1997; 25:745–6.

48. Isenberg SF. Thyroid abscess resulting from fine-needle aspiration. Otolaryngol Head Neck Surg. 1994; 111:832–3.

49. Nishihara E, Miyauchi A, Matsuzuka F, Sasaki I, Ohye H, Kubota S, et al. Acute suppurative thyroiditis after fine-needle aspiration causing thyrotoxicosis. Thyroid. 2005; 15:1183–7.

50. Sun JH, Chang HY, Chen KW, Lin KD, Lin JD, Hsueh C. Anaerobic thyroid abscess from a thyroid cyst after fine-needle aspiration. Head Neck. 2002; 24:84–6.

51. Unluturk U, Ceyhan K, Corapcioglu D. Acute suppurative thyroiditis following fine-needle aspiration biopsy in an immunocompetent patient. J Clin Ultrasound. 2014; 42:215–8.

Figure 1.
Flow diagram of study screening and selection for the present review. FNAB, fine-needle aspiration biopsy.
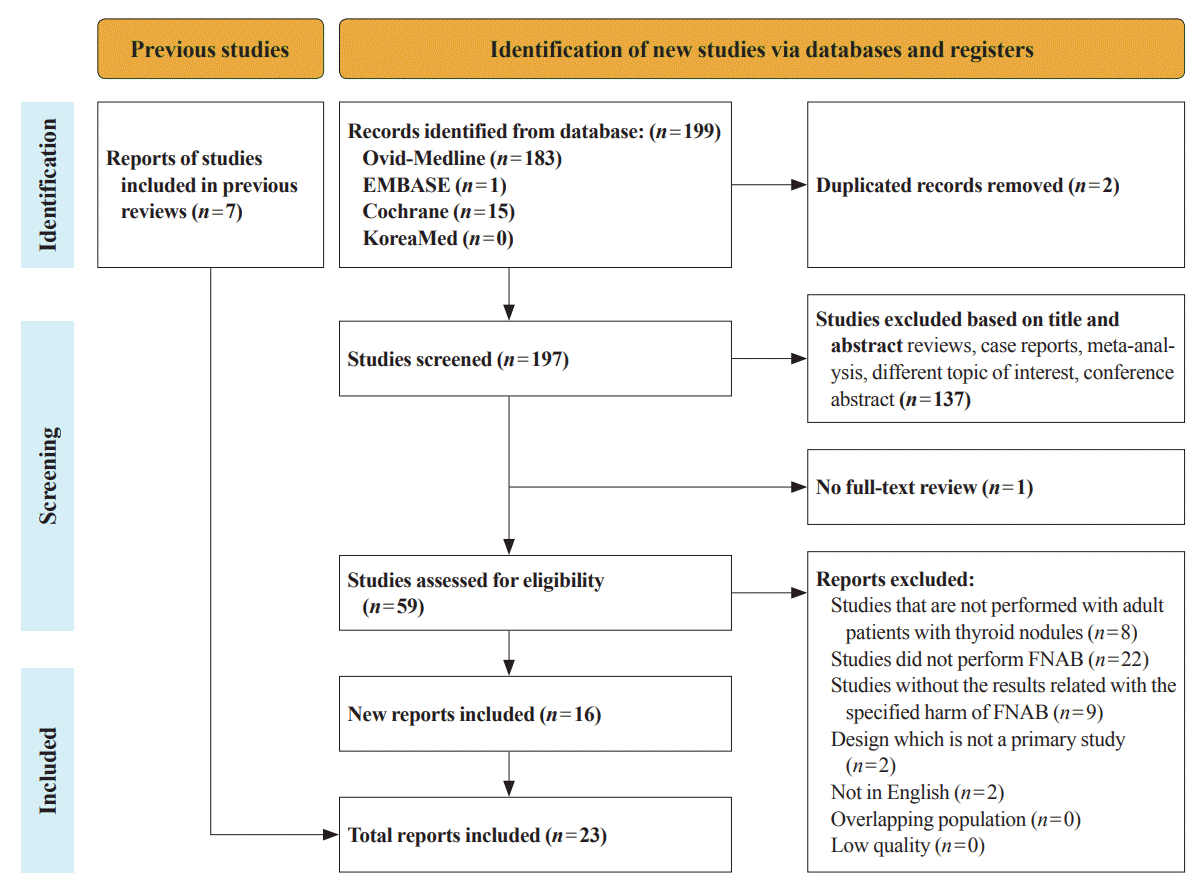
Table 1.
Summary of Studies Related to the Harms of Ultrasonography-Guided Fine-Needle Aspiration Biopsy for Thyroid Neoplasms
| Study | Country recruitment period | Study design | No. of cases/patients | Age, yr | Female, % | Needle gauge | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Pain after FNA | ||||||||
| Lee et al. (2019) [12] | South Korea 2018 | Randomized, double-blind | 99/99 (23G n=49, 25G n=50) | 51.5 | 79.8 | 23, 25 | Rated with NRSa | |
| 23G: none 3 (6.1%), mild 39 (79.6%), moderate 6 (12.2%), severe 1 (2.0%) | ||||||||
| 25G: none 10 (20%), mild 36 (72.0%), moderate 4 (8.0%), severe 0 (0.0%) | ||||||||
| Jung et al. (2018) [13] | South Korea 2017 | Retrospective | 88/88 (21G n=38, 23G n=50) | 54.6 | 84.1 | 21, 23 | Rated with NRSa | |
| 21G: none 1 (2.6%), mild 33 (86.8%), moderate 4 (10.5%), severe 0 (0.0%) | ||||||||
| 23G: none 6 (12.0%), mild 42 (84.0%), moderate 2 (4.0%) severe 0 (0.0%) | ||||||||
| Cordes et al. (2018) [14] | Germany 2017–2018 | Prospective | 205/205 | 55 | 71.2 | 22, 24 | Pain at the puncture site 5 (2.4%) | |
| Birgi et al. (2016) [11] | Turkey 2014 | Prospective | 138/138 | 49.4 | 88.4 | 21 | No pain 33.3% | |
| Pain 66.6% (low 48.3% medium 15.7%, high 2.7%) | ||||||||
| Toman et al. (2016) [15] | Turkey 2012 | Retrospective | 98/98 | 49.8 | 82.7 | 23 | 100-mm VAS ≥30 (70.5%) | |
| A higher pain score correlated with the nodule depth (r=0.43, P<0.001) | ||||||||
| Lee et al. (2013) [16] | South Korea 2012 | Retrospective | 157/157 (experienced radiologist n=75, less experienced radiologist n=82) | 51.4 | 89.2 | 23 | Rated with NRSa | |
| Experienced radiologist: none 14 (18.7%), mild 48 (64.0%), moderate 11 (14.7%), severe 3 (4.0%) | ||||||||
| Less experienced radiologist: none 12 (14.6%), mild 48 (58.5%), moderate 20 (24.4%), severe 5 (6.1%) | ||||||||
| Kim et al. (2012) [10] | South Korea 2007–2009 | Retrospective | 1,456/977 | 49.0 | 85.8 | 23 | Mild pain 87 (8.9%) | |
| No other major complications | ||||||||
| Gursoy et al. (2007) [18] | Turkey Apr.–Sep. 2006 | Randomized, double-blind | 99/99 (placebo n=49, EMLA n=50) | 46.8 | 19.5 | 25 | Rated with VRS | |
| Placebo group: none 4, mild 16 (32.7%), moderate 14 (28.6%), severe 15 (30.6%) | ||||||||
| EMLA group: none 9 (18%), mild 26 (52%), moderate 11 (22%), severe 4 (8%) | ||||||||
| Gursoy et al. (2007) [17] | Turkey Aug.–Dec. 2006 | Randomized, double-blind | 107/107 (placebo n=52, lidocaine n=55) | 46.5 | 15.6 | 25 | Rated with VRS | |
| Placebo group: none 6 (11.5%), mild 17 (32.7%), moderate 14 (26.9%), severe 15 (28.8%) | ||||||||
| Lidocaine group: none 23 (41.8%), mild 27 (49.1%), moderate 5 (9.1%), severe 0 (0%) | ||||||||
| Hematoma or any bleeding events after the procedure | ||||||||
| Ahn et al. (2021) [22] | South Korea 2010–2014 | Retrospective | 627/583 | 49.6 | 67.5 | 23 | Comparison with the CNB group | |
| FNAB group 0 (0.0%) | ||||||||
| Kim et al. (2019) [23] | South Korea 2015 | Retrospective | 87/87 | 52.5 | 87.4 | 26 | Subcapsular hematoma 3 (3.4%) | |
| Khadra et al. (2018) [28] | USA 2008–2016 | Retrospective | 1,568/802 | 63.0 (AT/AC) | 80.2 | 25 | Total 9/1,568 (0.6%) | |
| 50.1 (control) | AT/AC 3/336 (0.9%) | |||||||
| No agent 6/1,232 (0.5%) | ||||||||
| Jung et al. (2018) [13] | South Korea 2017 | Retrospective | 88/88 | 54.6 | 84.1 | 21, 23 | Intrathyroidal 1 (2.6%) | |
| Cordes et al. (2018) [14] | Germany 2017–2018 | Prospective | 205/205 | 55 | 71.2 | 22, 24 | Minor hematomas 2 (0.9%) | |
| Kavanagh et al. (2017) [19] | Ireland 2006–2013 | Retrospective | 724/724 | 40 | 82.5 | 20, 23 | Post-procedural 6 (0.8%) | |
| Chae et al. (2017) [24] | South Korea 2013–2015 | Retrospective | 5,121/5,121 | 50.9 | 78.8 | 23 | Comparison with the CNB group FNAB group 43 (0.8%) | |
| Cappelli et al. (2017) [21] | Italy 2007–2016 | Retrospective | 7,449/6,323 | 54.3 | 81.0 | 25 | Intrathyroidal 4 (0.06%) | |
| Carotid intramural 1 (0.02%) | ||||||||
| Birgi et al. (2016) [11] | Turkey 2014 | Prospective | 138/138 | 49.4 | 88.4 | 21 | Minor hematoma 1 (0.7%) | |
| Uchida et al. (2016) [27] | Japan 2011–2013 | Retrospective | 742/653 | 59.0 | 74.1 | 22 | Acute thyroid swelling or anechoic lesions 8 (1.2%) | |
| Chen et al. (2015) [26] | USA 2007–2011 | Retrospective | 96/96 | 56.0 | 83.0 | 25, 27 | Local hematoma 1 (1.0%) | |
| Lee et al. (2013) [16] | South Korea 2012 | Retrospective | 157/157 | 51.4 | 89.2 | 23 | Small intraglandular hematoma 1 (0.6%) | |
| Abu-Yousef et al. (2011) [29] | USA 2006–2007 | Retrospective | 788/593 | NR | NR | 22, 25 | Including AT/AC patients | |
| AT/AC 2 (1.4%) | ||||||||
| Control 4 (0.9%) | ||||||||
| Khoo et al. (2008) [25] | USA 1999–2001 | Retrospective | 311/311 | 54.6 | 74.3 | NR | Compared with FNAB+CNB group | |
| FNAB-only group 3 (1%) | ||||||||
| Newkirk et al. (2000) [20] | USA 1996–1998 | Retrospective | 234/215 | 51.9 (female) | 82.9 | 22, 23, 25 | Perithyroidal or superficial 15 (6.4%) | |
| 57.8 (male) | ||||||||
| Neurological symptoms | ||||||||
| Cordes et al. (2018) [14] | Germany 2017–2018 | Prospective | 205/205 | 55 | 71.2 | 22, 24 | Total complications 9 (4.4%) | |
| Paresthesia 8 (3.9%) | ||||||||
| Dysphonia 1 (0.5%) | ||||||||
| Kavanagh et al. (2017) [19] | Ireland 2006–2013 | Retrospective | 724/724 | 40 | 82.5 | 20, 23 | Vasovagal reaction 2 (0.3%) | |
| Cappelli et al. (2017) [21] | Italy 2007–2016 | Retrospective | 7,449/6,323 | 54.3 | 81.0 | 25 | Vasovagal reaction 1 (0.02%) | |
| Tomoda et al. (2006) [30] | Japan 2004–2005 | Retrospective | 10,974/10,974 | NR | NR | 23 | Transient vocal cord paralysis 4 (0.04%) | |
| Newkirk et al. (2000) [20] | USA 1996–1998 | Retrospective | 234/215 | 51.9 (female) | 82.9 | 22, 23, 25 | Hoarseness (transient) 2 (0.9%) | |
| 57.8 (male) | Vasovagal reaction 3 (1.3%) | |||||||
| Tracheal puncture during the procedure | ||||||||
| Cappelli et al. (2017) [21] | Italy 2007–2016 | Retrospective | 7,449/6,323 | 54.3 | 81.0 | 25 | Tracheal puncture 2 (0.04%) | |
| Needle tract implantation of thyroid cancer | ||||||||
| Hayashi et al. (2020) [33] | Japan 2006–2017 | Retrospective | 11,745/11,745 | 70.6 | NR | NR | NTI in thyroid 22 (0.19%) | |
| Cappelli et al. (2017) [21] | Italy 2007–2016 | Retrospective | 7,449/6,323 | 54.3 | 81.0 | 25 | Cancer seeding along the track of the needle 1 (0.02%) | |
| Ito et al. (2005) [31] | Japan 1990–2002 | Retrospective | 4,912/4,912 | NR | NR | 22 | Needle tract implantation of papillary thyroid carcinoma 7 (0.14%) | |
FNA, fine-needle aspiration; NRS, numeric rating scale; VAS, visual analogue scale; EMLA, eutectic mixture of local anesthetics; VRS, verbal rating scale; CNB, core needle biopsy; FNAB, fine-needle aspiration biopsy; AT/AC, antithrombotic/anticoagulant agent; NR, not reported; NTI, needle tract implantation.
Table 2.
Key Points for Reducing FNAB-Related Complications




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download