1. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011; 365:1713–1725.

2. Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011; 140:1785–1794.

3. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012; 142:46–54.

4. Loftus CG, Loftus EV Jr, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007; 13:254–261.

5. Yang SK, Loftus EV Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease in Asia. Inflamm Bowel Dis. 2001; 7:260–270.

6. Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006; 12:6102–6108.

7. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119.

8. Yen HH, Weng MT, Tung CC, et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide populationbased study. Intest Res. 2019; 17:54–62.

9. Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012; 27:1266–1280.

10. Jung YS, Han M, Kim WH, Park S, Cheon JH. Incidence and clinical outcomes of inflammatory bowel disease in South Korea, 2011-2014: a nationwide population-based study. Dig Dis Sci. 2017; 62:2102–2112.

11. Park SH, Kim YJ, Rhee KH, et al. A 30-year trend analysis in the epidemiology of inflammatory bowel disease in the Songpa-Kangdong district of Seoul, Korea in 1986-2015. J Crohns Colitis. 2019; 13:1410–1417.

12. Lee JH, Cheon JH, Kim ES, et al. The prevalence and clinical significance of perinuclear anti-neutrophil cytoplasmic antibody in Korean patients with ulcerative colitis. Dig Dis Sci. 2010; 55:1406–1412.

13. Park SH, Kim YM, Yang SK, et al. Clinical features and natural history of ulcerative colitis in Korea. Inflamm Bowel Dis. 2007; 13:278–283.

14. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012; 142:257–265.

15. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987; 317:1625–1629.

16. Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011; 60:780–787.

17. Suzuki Y, Motoya S, Hanai H, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014; 49:283–294.

18. Muñoz-Villafranca C, Ortiz de Zarate J, Arreba P, et al. Adalimumab treatment of anti-TNF-naïve patients with ulcerative colitis: deep remission and response factors. Dig Liver Dis. 2018; 50:812–819.

19. Bálint A, Farkas K, Palatka K, et al. Efficacy and safety of adalimumab in ulcerative colitis refractory to conventional therapy in routine clinical practice. J Crohns Colitis. 2016; 10:26–30.

20. Dulai PS, Siegel CA, Colombel JF, Sandborn WJ, Peyrin-Biroulet L. Systematic review: monotherapy with antitumour necrosis factor α agents versus combination therapy with an immunosuppressive for IBD. Gut. 2014; 63:1843–1853.

21. Taxonera C, Iglesias E, Muñoz F, et al. Adalimumab maintenance treatment in ulcerative colitis: outcomes by prior antiTNF use and efficacy of dose escalation. Dig Dis Sci. 2017; 62:481–490.

22. Hussey M, Mc Garrigle R, Kennedy U, et al. Long-term assessment of clinical response to adalimumab therapy in refractory ulcerative colitis. Eur J Gastroenterol Hepatol. 2016; 28:217–221.

23. Colombel JF, Jharap B, Sandborn WJ, et al. Effects of concomitant immunomodulators on the pharmacokinetics, efficacy and safety of adalimumab in patients with Crohn’s disease or ulcerative colitis who had failed conventional therapy. Aliment Pharmacol Ther. 2017; 45:50–62.

24. Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn’s disease: a prospective, randomized trial. J Crohns Colitis. 2016; 10:1259–1266.

25. Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013; 19:2118–2124.

26. Dahiya DS, Kichloo A, Wani F, Singh J, Solanki D, Shaka H. A nationwide analysis on the influence of obesity in inflammatory bowel disease hospitalizations. Intest Res. 20:342–349.

27. Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease: results from a prospective population-based study. Gut. 2008; 57:1518–1523.

28. Iwasa R, Yamada A, Sono K, Furukawa R, Takeuchi K, Suzuki Y. C-reactive protein level at 2 weeks following initiation of infliximab induction therapy predicts outcomes in patients with ulcerative colitis: a 3 year follow-up study. BMC Gastroenterol. 2015; 15:103.

29. Con D, Andrew B, Nicolaides S, van Langenberg DR, Vasudevan A. Biomarker dynamics during infliximab salvage for acute severe ulcerative colitis: C-reactive protein (CRP)-lymphocyte ratio and CRP-albumin ratio are useful in predicting colectomy. Intest Res. 2022; 20:101–113.

30. Ardizzone S, Cassinotti A, Duca P, et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011; 9:483–489.

31. Lee KM, Jeen YT, Cho JY, et al. Efficacy, safety, and predictors of response to infliximab therapy for ulcerative colitis: a Korean multicenter retrospective study. J Gastroenterol Hepatol. 2013; 28:1829–1833.

32. Italian Group for the Study of Inflammatory Bowel Disease, Armuzzi A, Biancone L, et al. Adalimumab in active ulcerative colitis: a “real-life” observational study. Dig Liver Dis. 2013; 45:738–743.

33. Taxonera C, Estellés J, Fernández-Blanco I, et al. Adalimumab induction and maintenance therapy for patients with ulcerative colitis previously treated with infliximab. Aliment Pharmacol Ther. 2011; 33:340–348.

34. Van de Vondel S, Baert F, Reenaers C, et al. Incidence and predictors of success of adalimumab dose escalation and de-escalation in ulcerative colitis: a real-world Belgian cohort study. Inflamm Bowel Dis. 2018; 24:1099–1105.

35. Wolf D, D’Haens G, Sandborn WJ, et al. Escalation to weekly dosing recaptures response in adalimumab-treated patients with moderately to severely active ulcerative colitis. Aliment Pharmacol Ther. 2014; 40:486–497.

36. Lin WC, Wong JM, Tung CC, et al. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J Gastroenterol. 2015; 21:13566–13573.

37. Lee SH, Kim MJ, Chang K, et al. Fecal calprotectin predicts complete mucosal healing and better correlates with the ulcerative colitis endoscopic index of severity than with the Mayo endoscopic subscore in patients with ulcerative colitis. BMC Gastroenterol. 2017; 17:110.
38. Kristensen V, Røseth A, Ahmad T, Skar V, Moum B. Fecal calprotectin: a reliable predictor of mucosal healing after treatment for active ulcerative colitis. Gastroenterol Res Pract. 2017; 2017:2098293.

39. Yarur AJ, Jain A, Hauenstein SI, et al. Higher adalimumab levels are associated with histologic and endoscopic remission in patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2016; 22:409–415.

40. Bodini G, Giannini EG, Savarino V, et al. Adalimumab trough serum levels and anti-adalimumab antibodies in the long-term clinical outcome of patients with Crohn’s disease. Scand J Gastroenterol. 2016; 51:1081–1086.

41. Paul S, Moreau AC, Del Tedesco E, et al. Pharmacokinetics of adalimumab in inflammatory bowel diseases: a systematic review and meta-analysis. Inflamm Bowel Dis. 2014; 20:1288–1295.
42. Papamichael K, Baert F, Tops S, et al. Post-induction adalimumab concentration is associated with short-term mucosal healing in patients with ulcerative colitis. J Crohns Colitis. 2017; 11:53–59.

43. Ogata H, Hagiwara T, Kawaberi T, Kobayashi M, Hibi T. Safety and effectiveness of adalimumab in the treatment of ulcerative colitis: results from a large-scale, prospective, multicenter, observational study. Intest Res. 2021; 19:419–429.

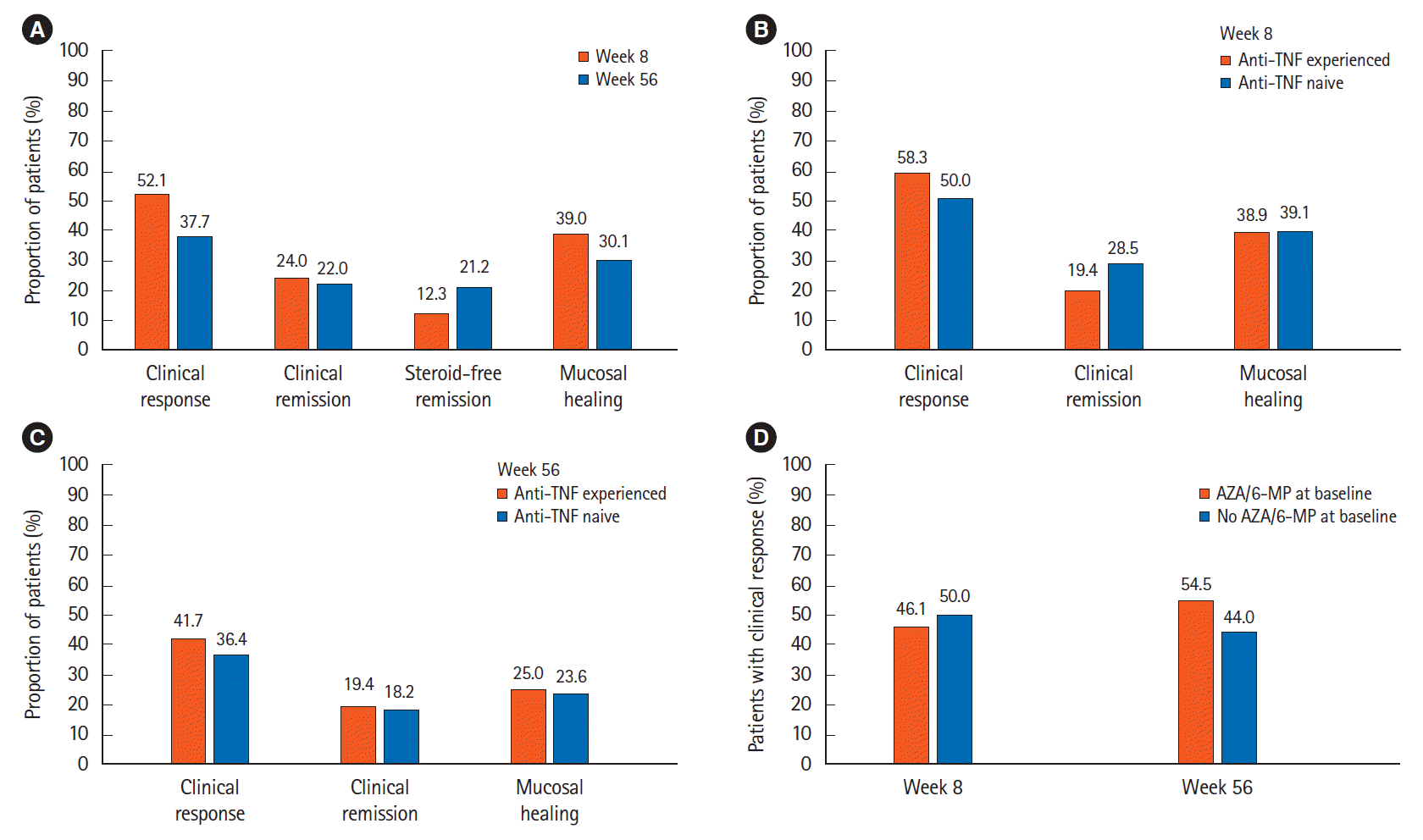
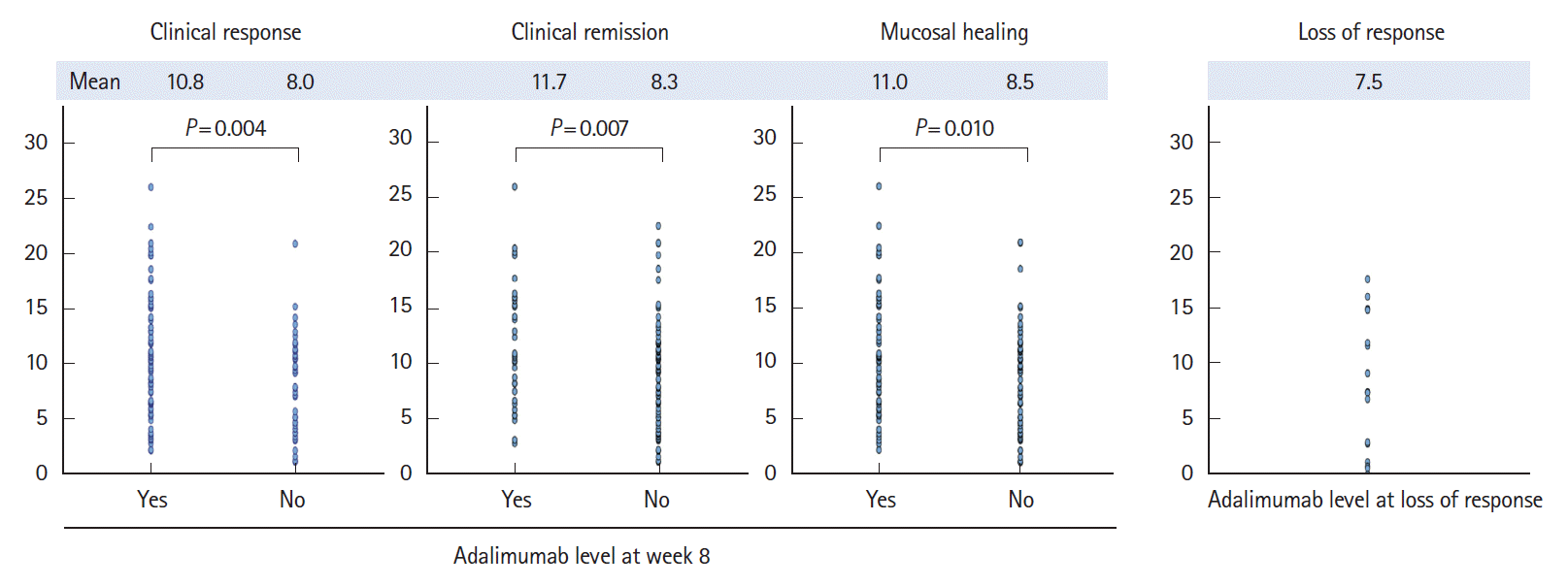
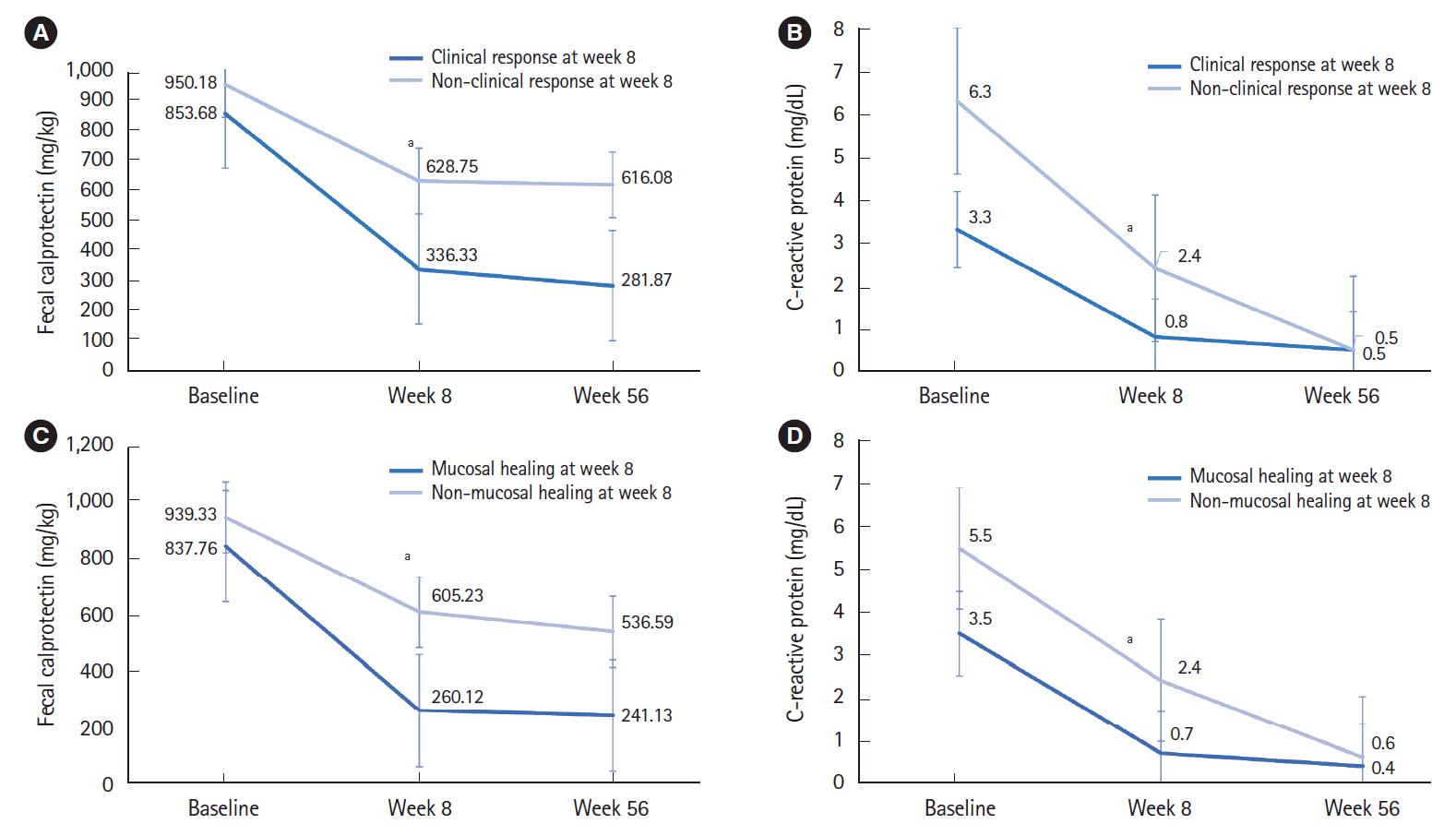
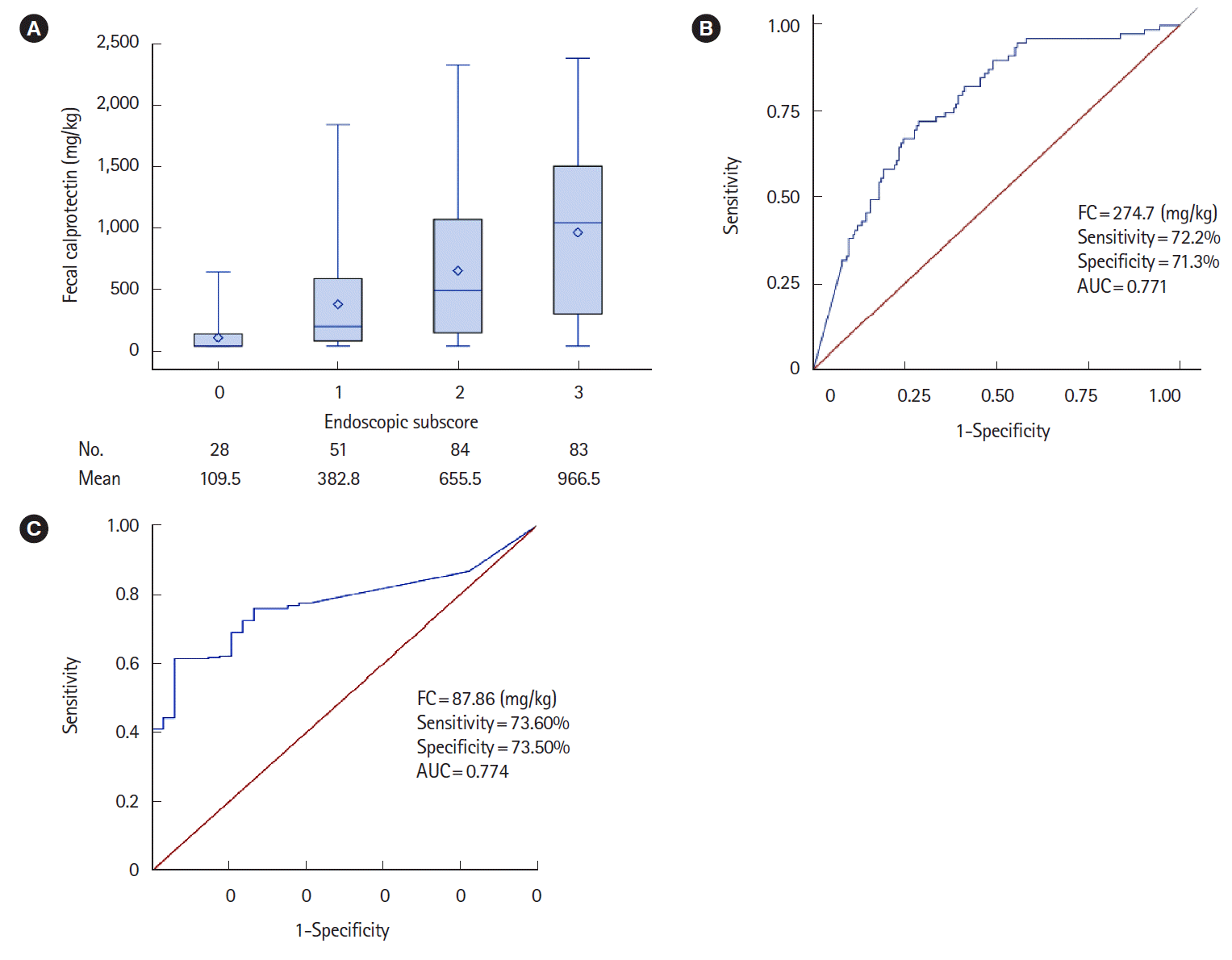




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download