Abstract
Background/Aims
Tight control of inflammation and adjustment of treatment if activity persists is the current strategy for the management of Crohn’s disease (CD). The usefulness of fecal calprotectin (FC) in isolated involvement of the small intestine in CD is controversial. To assess the usefulness of FC to determine the inflammatory activity detected by intestinal ultrasonography (IUS) in ileal CD.
Methods
Patients with exclusively ileal involvement CD who underwent IUS and an FC were prospectively included. Simple ultrasound index was used to determine inflammatory activity. The usual statistical tests for comparison of diagnostic techniques were used.
Results
One hundred and five patients were included, IUS showed inflammatory activity in 59% of patients and complications in 18.1%. FC showed a significant correlation with IUS in the weak range (Spearman coefficient r=0.502; P<0.001); the area under the receiver operating characteristic curve was 0.79 (95% confidence interval, 0.70–0.88; P<0.001). The FC value that best reflected the activity in IUS was 100 μg/g with sensitivity, specificity, and positive and negative predictive values of 73.0%, 71.4%, 79.3% and 63.8%, respectively. There were no differences in FC concentration between patients with or without transmural complications. The addition of serum C-reactive protein to FC did not improve the ability to assess IUS activity.
Conclusions
FC has a significant correlation with IUS to monitor ileal CD activity. This correlation is weak and it does not allow assessing the presence of CD complications. Both tests should be used in conjunction for tight control of ileal CD. More studies on noninvasive tests in this location are needed.
The therapeutic strategy of Crohn’s disease (CD) is changing in recent years. Strategy based solely on the control of the symptoms of the disease, probably, fails to change the natural course of the CD [1]. Since 2015, the treat to target strategy implies the identification of a predefined goal in the context of the patient’s individual needs to be achieved by the treatment, followed by regular monitoring (tight control) and therapy modifications if needed, until the goal is achieved [2]. The goal of mucosal healing has shown better outcomes, long-term steroid-free remission and lower rate of surgeries [3]. However, for the evaluation of mucosal healing endoscopies are necessary, but due to the invasive nature of the technique, the saturation of the endoscopy units, the cost of the exploration and the fact, that in a considerable percentage of patients, CD affects intestinal segments that are out of access of conventional endoscopy, this strategy is hardly applicable in daily clinical practice [2]. For this reason, there is currently great interest in the noninvasive tests to monitor CD. Cross-sectional imaging techniques and biomarkers (serum and fecal) are the most commonly noninvasive tests used today in clinical practice [4,5]. Magnetic resonance enterography (MRE) is used in many centers to monitor CD, but its cost, difficult accessibility and the need for unpleasant preparation make this technique difficult to use it repeatedly [4]. Intestinal ultrasonography (IUS) is cheap, no preparation is required and is a noninvasive and accessible exploration [4]; it has also shown its usefulness in monitoring endoscopic response after treatment in CD [6]. A simple index based on wall thickness and color Doppler grade has shown high accuracy in detecting endoscopic activity in patients with CD [7]. Recently, transmural healing in IUS has been associated with better long-term outcomes [8].
Calprotectin is a calcium and zinc binding protein, present in the cytoplasm of the neutrophils [2]. Its determination in feces is a sensitive marker of endoscopic activity in CD [5]. Furthermore, the elevation of their fecal concentration in asymptomatic patients has been correlated with the risk of relapse [9]. Recently, the CALM study has shown promising results in patients with CD, using fecal calprotectin (FC) and serum C-reactive protein (CRP) as a treatment target (tight control group). In 244 CD patients, treatment escalation of adalimumab was based on FC and serum CRP in 122 patients versus only clinical assessment in the other 122 CD patients. At week 48, mucosal healing was in significant higher proportion in the tight control group [10].
However, ileal CD patients represent a population at-risk in whom treat to target, disease monitoring and therapy adjustment cannot be easily applied [11]. There are conflicting results regarding the ability of FC to assess the inflammatory activity of CD in relation their different locations [12]. Several studies [12,13] have shown lower levels of FC when the disease affects the small intestine and a systematic review on this topic from 2019 concluded that no firm conclusion can be made [14]. Several studies have assessed the correlation between FC and MRE to assess inflammation in ileal CD [15-19]. But firm conclusions cannot be drawn either due to the inclusion of patients with ileocolonic disease and the disparity of the results. Some authors have found a significant correlation [15,16,19] between calprotectin and the inflammatory activity of CD localized in the small intestine but this has not been confirmed by others [17,18]. On the other hand, as far as we know, the correlation between FC and ultrasonographic activity of CD has not yet been evaluated.
The objective of the study is to assess the correlation between FC and the inflammatory activity as detected by the IUS in patients with CD exclusively located in the small intestine.
This was an observational, cross-sectional clinical practice study. Between January 2019 and March 2020, we consecutively included all patients with a diagnosis of CD [20] and exclusively ileal involvement who underwent follow-up in our outpatient clinics. After reviewing the clinical history, all patients without colon involvement in the endoscopy were selected. Patients with ileal recurrence at endoscopy were also included. In addition, all patients had to have involvement of a segment of the small intestine on IUS. The exclusion criteria were: patients under 18 years of age, pregnant, nonsteroidal anti-inflammatory treatment and refusal of the patient to participate in the study.
All patients included in the study should have accomplished our center’s follow-up protocol for patients with CD. Routine follow-up consists of scheduled outpatient visits every 3 to 6 months or every 12 months depending on the clinical situation and/or treatment of each patient. Each visit includes clinical assessment with the Harvey-Bradshaw index (it is considered inactive with a score equal to or less than 4 points) [21], evaluations of routine analysis results, including biomarkers used in routine practice to determine the presence of inflammatory activity (FC and serum CRP) and IUS examination, which is performed as control of morphologic inflammatory activity in patients with CD. IUS assessment is performed in our center every 3 to 6 months if the inflammatory activity persists or every 12 months in patients with deep remission. With this protocol, all CD patients are evaluated and have an IUS examination at least once a year. Determination of FC and analytical extraction were carried out in a period of less than 4 weeks after or before the scheduled IUS examination within their follow-up protocol. No changes were allowed in the therapeutic regimen during the time between the 2 procedures in order to be included in the study. For this study, we have considered IUS as the reference technique, given the experience gained after years of monitoring CD and that FC was subsequently used in our center.
For the correct collection of the FC sample, all patients were given the following instructions: collect the first stool sample of the day, avoid highly liquid or too solid stools, after collecting the samples can be kept frozen [22]. In our center, FC is determined by immunoassay technique that is time-resolved fluorimetry using a lanthanide chelate (europium). The autoanalyzer is the AQT90 (Radiometer, Copenhagen, Denmark). Its result is expressed in micrograms per gram of stool: normal limits: < 50 µg/g negative; 50–100 µg/g indeterminate; and > 100 µg/g positive.
IUS was performed using an ultrasound unit (Aplio 80; Toshiba Medical Systems Corp., Tokyo, Japan), initially with a 3–6 MHz transducer and then with a 6–10 MHz probe for a detailed evaluation. Patients had fasted overnight, and no bowel preparation was used [23].
The examinations were performed by 2 radiologists (T.R. and M.J.M.) with at least 10 years of experience in IUS. The radiologist was unaware of the clinical and laboratory findings.
The following features were assessed in the ultrasound examinations: (1) wall thickness of the affected segment: wall thickness > 3 mm was considered abnormal [24]; (2) vascularization of the wall using color Doppler, with classification into the following grades: grade 0 (absent vascularization), grade 1 (barely visible vascularization), grade 2 (moderately visible vascularization) and grade 3 (markedly visible vascularization) [25]; and (3) presence of complications (stenosis, fistulas, or inflammatory masses) [26]. Bowel wall vascularity was determined by color Doppler ultrasound with a special preset optimized for slow flow detection, was then evaluated (filter at low setting, 50 Hz, and lowest velocity scale, 2 cm/s), and was kept constant for all examinations. Color Doppler flow was considered present when color pixels persisted throughout the examination
To measure the disease activity, we use a simple ultrasound score previously published, based on parietal thickness (mm) +color Doppler grade [7]; 5.5 was the cutoff point employed to diagnose IUS inflammatory activity. This cutoff point has shown high accuracy for diagnosis of active disease (sensitivity 90%, specificity 86.4%, positive predictive value 93.8%, negative predictive value 79.2%, and accuracy 88.9%, receiver operating characteristic [ROC] area 0.923) [7].
We used basic descriptive statistics, which included the mean and standard deviation (SD) for continuous variables, and absolute frequency and percentages for discrete variables. Using the ROC curve, the calprotectin value that had the best accuracy to reflect ultrasound activity (simple ultrasound score > 5.5 points) was determined. Subsequently, the correlation between calprotectin and IUS activity was evaluated using the Spearman test. Mann-Whitney test was used to assess the association between the different ultrasound variables and FC. The IBM Statistical Package for the Social Sciences version 22.0.0; 2013 (IBM Corp., Armonk, NY, USA) was used to describe and analyze the data, considering P-values < 0.05 as significant.
During the inclusion period, 115 patients with ileal CD were identified. Ten patients were excluded: 2 for not having an IUS, 6 did not collect a valid stool sample, and 2 for more than 4 weeks between examinations (Fig. 1). The demographic and disease characteristics of the 105 patients included in the study are shown in Table 1. On the day of the outpatient visit, 31 patients (29.5%) had a Harvey-Bradshaw index greater than 4 points. Serum CRP values were greater than 10 mg/dL in 18 patients (17.1%) and FC was greater than 100 µg/g in 62 patients (59%).
The most widely used drugs were biologics. As monotherapy, biologics were prescribed in 36 patients (34.3%) and combined with an immunosuppressor drug in 12 patients (11.4%). Adalimumab was the most widely used, 25 patients (23.8%) were taking this drug at the time of the study.
Ultrasound findings are shown in Table 2. At the time of the study, IUS showed an intestinal wall thickness > 3 mm in 82 patients (78.1%); color Doppler grade 2 or 3 was detected in 42 patients (40%). IUS complications were found in 19 patients (18.1%): stenosis in 9 (1 patient had stenosis and fistula) and 10 patients showed abdominal fistulizing complications (5 isolated fistula, 2 with fistula and inflammatory mass, and 3 with a collection). The simple IUS index was greater than 5.5 points in 62 patients (59%).
The mean time between both examinations was 8.8 days (SD, 7.07). Only 21 patients (20%) had a time greater than 15 days between performing the ultrasound and collecting FC.
A significant correlation was found between FC levels and the inflammatory activity detected with IUS (simple score > 5.5 points), with a weak range (Spearman correlation coefficient [r] = 0.502; P<0.001). The mean concentration of FC was significantly higher in patients with simple IUS score activity compared to those without inflammatory activity on IUS, 381.14 µg/g (SD, 511.33) versus 121.29 µg/g (SD, 219.07) respectively using the Mann-Whitney U test (P<0.001). FC showed an area under the ROC curve (accuracy in diagnosing inflammatory activity using IUS) of 0.796 (95% confidence interval [CI], 0.70–0.88; P<0.001) (Fig. 2), with 100 µg/g as the best cutoff point; with a sensitivity, specificity, positive predictive value, and negative predictive value of 73.0% (95% CI, 61.0%–82.4%), 71.4% (95% CI, 56.4%–82.8%), 79.3% (95% CI, 67.2%–87.7%), and 63.8% (95% CI, 49.4%–76.0%), respectively.
Table 3 shows the comparison of FC results according to the values of the different IUS variables. Patients with intestinal wall thickness > 3 mm, color Doppler grade 2–3, and an intestinal affected length greater than 20 cm had a significantly higher concentration of FC. A value of FC > 100 µg/g showed a significant correlation with the different ultrasound variables, although with a very low strength correlation; the r was 0.4 (P<0.001) with wall thickness > 3 mm; 0.3 (P=0.002) with color Doppler grade 2–3 and 0.2 (P=0.03) with affected intestinal length > 20 cm. A significant linear correlation was evidenced between the wall thickness and the increase in FC concentration (Fig. 3). An increase of 1 mm in the intestinal wall thickness led to an increase of 67.1 μg in the concentration of FC (95% CI, 31.8–102.4).
All patients with some complication in IUS, showed a simple IUS index higher than 5.5 points, indicating the presence of inflammatory activity. No significant differences were found when comparing the mean of FC concentration between patients with ultrasound activity versus patients with inflammatory activity and complication in IUS (Table 3).
Only 4 patients had serum CRP greater than 10 mg/dL and FC less than 100 µg/g. The combination of both variables did not improve the results of FC to assess ultrasound inflammatory activity. The correlation between FC > 100 µg/g and CRP > 10 mg/dL and the simple IUS index was significant, but in a weak rank (r=0.46, P<0.001), area under the ROC curve 0.76, sensitivity 77.8% (95% CI, 66.1%–86.3%), specificity 69.0% (95% CI, 54.0%–80.9%), positive predictive value 79.0% (95% CI, 67.4%–87.3%), and negative predictive value 67.4% (95% CI, 52.5%–72.5%).
Table 4 shows the usefulness of the Harvey-Bradshaw index and serum CRP to determine the inflammatory activity detected by IUS and FC. For both variables, the correlations, with IUS and FC were weaker (significant correlation but in the range of weak for the Harvey-Bradshaw index and null for CRP) than those obtained between IUS and FC.
The present study compares FC with IUS to monitor inflammatory activity during follow-up of patients with CD exclusively localized in the small intestine. Our results show that this biomarker shows a significant correlation, although weak, with disease activity in patients with small bowel CD, when evaluated according to the IUS score.
We have analyzed the most appropriate cutoff value of FC for the assessment of inflammation with IUS and have found to be 100 µg/g, that showed a sensitivity and specificity of 73% and 74.1%, respectively. This would imply, if we only use 1 of the 2 tests, that of the 63 patients with inflammatory activity detected on IUS, 17 had not been detected based on the FC figures. That is, treatment would not have been modified in 27% of the patients based on the FC, but it would be modified according to the IUS.
Tight control of objective inflammatory activity allows us to adjust the treatment in CD to achieve better results. However, it remains to be defined which tests (endoscopic, radiological or serum and fecal biomarkers) and how often to perform them, to assess the inflammatory activity in CD [2]. In addition, when CD exclusively affects the small intestine, there is a higher frequency of complications, worse response to treatment, a higher rate of surgery and more difficult monitoring than colonic CD [11,27]. The difficulty of accessing the involved segment with conventional endoscopes, when CD is located in the small intestine, together with the limitation that stenoses supposed to use the endoscopic capsule, the difficulties in performing an enteroscopy (lack of accessibility and impossibility of a deep insertion due to stenosis, adhesions and the risk of perforation) [27] and the contradictory results shown by the use of FC to assess inflammatory activity in this location [4], make imaging techniques a fundamental tool to monitor activity CD in the small intestine.
Although, other studies have assessed the accuracy of FC to evaluate the CD activity detected with cross-sectional imaging techniques, only 1 study in the pediatric population has previously used IUS [28]. The objective of this study was not to evaluate the usefulness of FC; but to determine the changes produced by infliximab treatment in IUS and biomarkers during the follow-up of 28 children with ileal CD. In this work, a strong correlation was shown between FC and the different ultrasound variables, mainly with color Doppler (r=0.63, r=0.71, and r=0.61 between calprotectin and bowel wall thickness, color Doppler signal and length of intestinal involvement respectively). However, these authors did not use an index to determine the presence of global activity in the IUS. Our work also showed a correlation between FC and the different ultrasound variables, although weaker than in this work.
Among the authors who have assessed the relationship between FC and CD activity with cross-sectional imaging techniques, the majority have used MRE and different activity scores of this technique [15-18]. The work of Cerrillo et al. [16] compared FC with the magnetic resonance index of activity. This study showed better results than those obtained in our work, with a moderate correlation (r=0.56), higher cutoff point of FC (188 µg/g) and a sensitivity of 90%. However, this work included some patients with ileocolonic involvement (29.2%), which may justify their better results. Our results agree with those of Makanyanga et al. [15] they also found a weak correlation (r=0.4) between FC and a global MRE index. In this study, the mean FC was higher among patients with ileocolonic and colonic disease than in those with isolated ileal involvement. Other authors found no correlation between FC and MRE activity [17,18].
In our work, FC was not higher among patients with the presence of a complication in the IUS compared to those who only presented signs of inflammatory activity in the IUS (Table 3). We did not assess the differences in FC concentration between the different types of complications, given the small number included. Cerrillo et al. [16] showed differences in FC concentration among patients with abdominal fistula in MRE; although not among those patients with and without abscess. However, they compared patients with complications versus patients without complications regardless of the presence of inflammatory activity in the MRE. We have compared patients with activity in IUS versus those with complications in IUS, since all patients with complication presented an index higher than 5.5 points indicative of inflammatory activity.
In our study, the sum of the CRP and FC values did not improve the ability to assess the presence of ultrasound activity. Although in some studies the combination of FC and CRP has been shown to be superior to assess endoscopic activity [29], the CALM study [10] using both biomarkers (FC in combination with serum CRP) to decide to intensify treatment at week 35 of follow-up, both biomarkers showed the same contribution (45% of patients) in the decision to escalate when they were assessed separately. In this study, only 25% of the patients, distributed between the tight control group and the standard treatment (12% and 17%, respectively), had isolated ileal disease; therefore their conclusions cannot be extrapolated to patients with ileal involvement without colonic disease. Other studies have not shown that the combination of CF and CRP improves the detection of endoscopic activity in CD located in the small intestine [30].
One of the limitations of the study is the difficulty of using a simple and validated index to determine IUS activity in clinical practice. For this reason, in this work, we have valued the activity of the IUS with the index that we use in our usual practice. Although this index has shown a good correlation with endoscopic activity in CD [7], it is not validated and does not include the administration of intravenous contrast. On the other hand, assessing the patients at a specific time of follow-up (88% with some type of treatment) does not allow taking into account the evolution of FC from baseline to the time of the study, which could better reflect its value in monitoring CD activity, given its variability.
Our cohort was consecutive and reasonably large, but it reflects our local population and may not be directly generalizable to other hospitals and patients. For example, only 4.7% of our population had an abscess, which may be different from other hospital settings.
In conclusion, FC has a significant correlation with IUS to monitor ileal CD activity. However, this correlation is weak and, furthermore, it does not allow assessing the complications of CD, therefore both tests should be used in conjunction for tight monitoring of ileal CD. More studies are needed on noninvasive tests in this location of CD.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contribution
Conceptualization: Paredes JM. Ultrasonography performed: Ripollés T, Martínez MJ. Laboratory analysis: Llopis P. Data collect and project administration: Paredes JM, Algarra A, Diaz R, Moreno N, Latorre P, López A. Writing original draft: Paredes JM. Writing-review and editing: Paredes JM, Ripollés T, Moreno-Osset E. Approval of final manuscript: all authors.
REFERENCES
1. Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2019; 68:423–433.

2. Gonczi L, Bessissow T, Lakatos PL. Disease monitoring strategies in inflammatory bowel diseases: what do we mean by “tight control”? World J Gastroenterol. 2019; 25:6172–6189.

3. Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther. 2016; 43:317–333.

4. Allocca M, Danese S, Laurent V, Peyrin-Biroulet L. Use of cross-sectional imaging for tight monitoring of inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020; 18:1309–1323.
5. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18:2218–2224.

6. Bryant RV, Friedman AB, Wright EK, et al. Gastrointestinal ultrasound in inflammatory bowel disease: an underused resource with potential paradigm-changing application. Gut. 2018; 67:973–985.

7. Ripollés T, Poza J, Suarez Ferrer C, Martínez-Pérez MJ, Martín-Algíbez A, de Las Heras Paez B. Evaluation of Crohn’s disease activity: development of an ultrasound score in a multicenter study. Inflamm Bowel Dis. 2021; 27:145–154.

8. Castiglione F, Imperatore N, Testa A, et al. One-year clinical outcomes with biologics in Crohn’s disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther. 2019; 49:1026–1039.

9. Zhulina Y, Cao Y, Amcoff K, Carlson M, Tysk C, Halfvarson J. The prognostic significance of faecal calprotectin in patients with inactive inflammatory bowel disease. Aliment Pharmacol Ther. 2016; 44:495–504.

10. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017; 390:2779–2789.

11. Dulai PS, Singh S, Vande Casteele N, et al. Should we divide Crohn’s disease into ileum-dominant and isolated colonic diseases? Clin Gastroenterol Hepatol. 2019; 17:2634–2643.

12. Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008; 14:40–46.

13. Gecse KB, Brandse JF, van Wilpe S, et al. Impact of disease location on fecal calprotectin levels in Crohn’s disease. Scand J Gastroenterol. 2015; 50:841–847.

14. Simon EG, Wardle R, Thi AA, Eldridge J, Samuel S, Moran GW. Does fecal calprotectin equally and accurately measure disease activity in small bowel and large bowel Crohn’s disease? A systematic review. Intest Res. 2019; 17:160–170.

15. Makanyanga JC, Pendsé D, Dikaios N, et al. Evaluation of Crohn’s disease activity: initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol. 2014; 24:277–287.

16. Cerrillo E, Beltrán B, Pous S, et al. Fecal calprotectin in ileal Crohn’s disease: relationship with magnetic resonance enterography and a pathology score. Inflamm Bowel Dis. 2015; 21:1572–1579.
17. Ye L, Cheng W, Chen BQ, et al. Levels of faecal calprotectin and magnetic resonance enterocolonography correlate with severity of small bowel Crohn’s disease: a retrospective cohort study. Sci Rep. 2017; 7:1970.

18. Zittan E, Kelly OB, Gralnek IM, Silverberg MS, Hillary Steinhart A. Fecal calprotectin correlates with active colonic inflammatory bowel disease but not with small intestinal Crohn’s disease activity. JGH Open. 2018; 2:201–206.

19. Jones GR, Fascì-Spurio F, Kennedy NA, et al. Faecal calprotectin and magnetic resonance enterography in ileal Crohn’s disease: correlations between disease activity and long-term follow-up. J Crohns Colitis. 2019; 13:442–450.

20. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989; 170:2–6.

21. Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010; 8:357–363.

22. Reenaers C, Bossuyt P, Hindryckx P, Vanpoucke H, Cremer A, Baert F. Expert opinion for use of faecal calprotectin in diagnosis and monitoring of inflammatory bowel disease in daily clinical practice. United European Gastroenterol J. 2018; 6:1117–1125.

23. Nylund K, Maconi G, Hollerweger A, et al. EFSUMB recommendations and guidelines for gastrointestinal ultrasound. Ultraschall Med. 2017; 38:273–284.
24. Fraquelli M, Colli A, Casazza G, et al. Role of US in detection of Crohn disease: meta-analysis. Radiology. 2005; 236:95–101.

25. Patriquin HB, Garcier JM, Lafortune M, et al. Appendicitis in children and young adults: Doppler sonographic-pathologic correlation. AJR Am J Roentgenol. 1996; 166:629–633.

26. Panés J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011; 34:125–145.

27. Watanabe K. Clinical management for small bowel of Crohn’s disease in the treat-to-target era: now is the time to optimize treatment based on the dominant lesion. Intest Res. 2020; 18:347–354.

28. Dillman JR, Dehkordy SF, Smith EA, et al. Defining the ultrasound longitudinal natural history of newly diagnosed pediatric small bowel Crohn disease treated with infliximab and infliximab-azathioprine combination therapy. Pediatr Radiol. 2017; 47:924–934.

29. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583.

Fig. 1.
Flowchart showing the number of patients entering the study. IUS, intestinal ultrasonography.
Fig. 2.
Fecal calprotectin receiver operating characteristic curve for assess inflammatory activity in intestinal ultrasonography of ileal Crohn´s disease. AUC, area under the receiver operating characteristic curve.
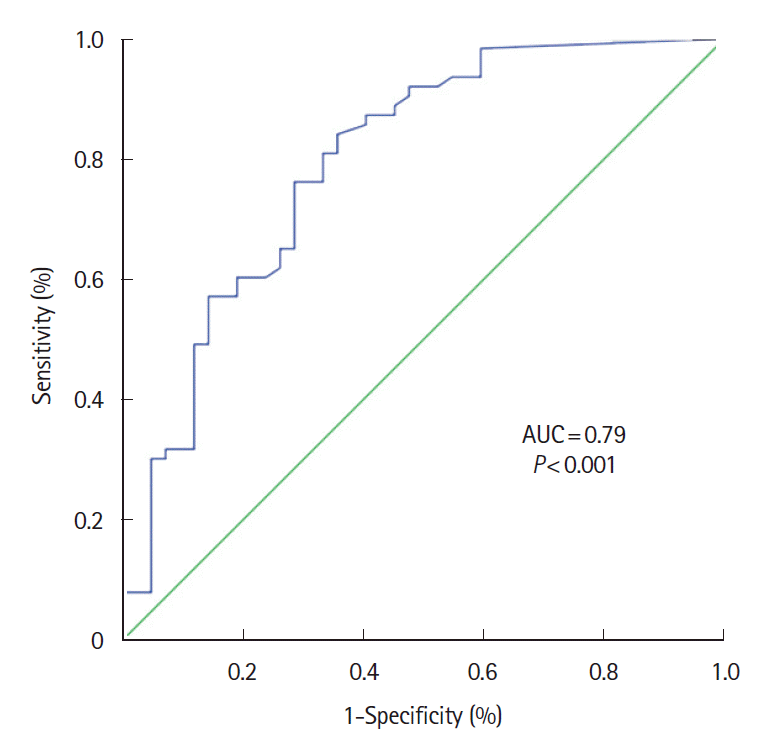
Fig. 3.
Correlation between fecal calprotectin and different ultrasound variables. (A) Correlation between simple ultrasound score and fecal calprotectin concentration. (B) Linear correlation between the wall thickness and fecal calprotectin concentration (95% confidence interval, CI).
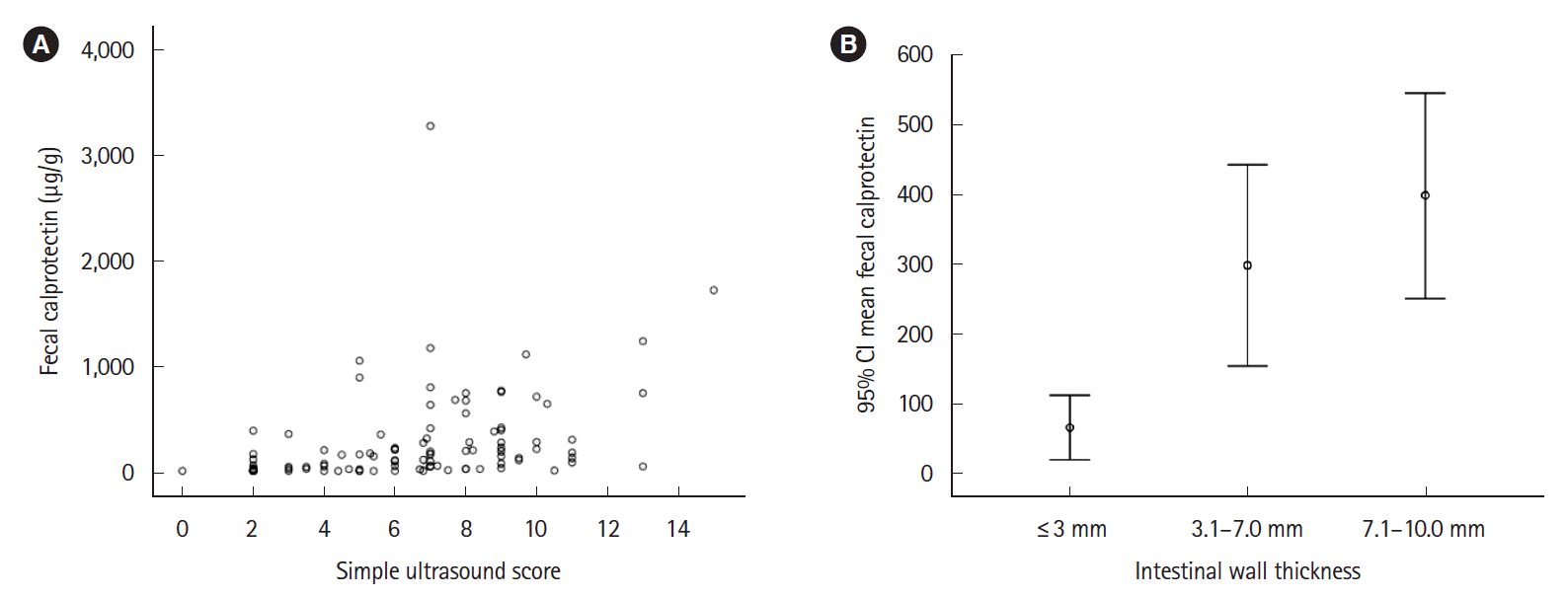
Table 1.
Demographic and Clinical Characteristics of the 105 Patients with Ileal Crohn’s Disease Included in the Study
Table 2.
Intestinal Ultrasonography Findings in 105 Patients with Ileal Crohn’s Disease Included in the Study
| Variable | Results |
|---|---|
| Wall thickness (mm), median (range) | 5 (2–13) |
| Patients with color Doppler grade 2 or 3, No. (%) | 42 (40.0) |
| Length (cm), median (range) | 8 (0–35) |
| Patients with transmural complications, No. (%)a | 19 (18.1) |
| Stenosis | 9 (8.6) |
| Abdominal fistula | 7 (6.6) |
| Abdominal collection | 5 (4.8) |
| Simple intestinal ultrasonography index (points), median (range) | 7 (0–15) |
Table 3.
Fecal Calprotectin to Assess Intestinal Ultrasonography Variables in 105 Patients with Ileal Crohn’s Disease Included in the Study
| Intestinal ultrasonography | Fecal calprotectin (µg/g) | P-valueb |
|---|---|---|
| Intestinal wall thickness | < 0.001 | |
| ≤ 3 mm | 66.00 ± 106.86 | |
| > 3 mm | 336.44 ± 475.51 | |
| Doppler grade | < 0.001 | |
| 0-1 | 171.49 ± 251.13 | |
| 2-3 | 435.76 ± 588.71 | |
| Affected intestinal length | 0.010 | |
| ≤ 20 cm | 250.79 ± 452.56 | |
| > 20 cm | 382.86 ± 359.68 | |
| Intestinal ultrasonography complication | 0.980 | |
| Noa | 347.47 ± 533.67 | |
| Yes | 459.70 ± 441.65 |
Table 4.
Relationship between the Different Noninvasive Variables Used to Monitor Inflammatory Activity in 105 Patiens with Isolated Ileal Crohn´s Disease Included in the Study




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download