Abstract
The timing of colonoscopy in patients with active ulcerative colitis (UC) lacks coherence. The published guidelines and recommendations advocate time-bound colonoscopy in patients with active UC to assess for mucosal healing. However, the practice of performing colonoscopies at fixed time frames lacks reasoning. The time to achieve mucosal healing in UC is not uniform across the patient populations and is influenced by the disease severity and efficacy and time to therapeutic response of the drugs being used. Additionally, with the availability of sensitive noninvasive inflammatory biomarkers such as fecal calprotectin, that parallel the disease activity and correlate with mucosal healing, the notion of performing colonoscopy at fixed intervals sounds unjustifiable. The authors express their view that a response-guided colonoscopy (driven by normalization of clinical symptoms and inflammatory biomarkers), rather than a time-bound colonoscopy, would be more logical, apart from being cost-effective and patient-friendly.
Endoscopy (colonoscopy or sigmoidoscopy) has a key role in the management of ulcerative colitis (UC). It provides crucial information for the diagnosis (and differential diagnosis), assessment of disease extent, activity and severity, evaluation of infections during relapse or development of new unexplained symptoms, surveillance for dysplasia/malignancy and documenting response to therapy [1-3]. With the availability of novel therapies for UC, the therapeutic targets have shifted from resolution of symptoms to achievement of mucosal healing (endoscopic or histologic) [4]. Achievement of mucosal healing is associated with a favorable disease course with reduced probability of relapse and lower risk of development of colorectal cancer [5,6]. Various practice guidelines for management of UC, therefore, suggest periodic endoscopic examinations to look for endoscopic and histologic disease activity [1-3].
For indications such as suspected relapse or surveillance for malignancy, endoscopic assessment is considered gold standard. However, ambiguity surrounds the timing and frequency of doing endoscopy for assessment of disease activity and mucosal healing after initiation of therapy. The Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE I and STRIDE II) programs initiated by the International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) suggest endoscopic mucosal assessment every 3–6 months after initiation of therapy [7,8]. The European Crohn’s and Colitis Organisation (ECCO) and the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) guidelines also recommend assessing clinical responders by endoscopy every 3–6 months, though the option of being guided by fecal calprotectin (FC) has also been propounded [1]. This time-bound repeated endoscopic assessment for mucosal healing is likely to result in very frequent invasive procedures which may be uncomfortable to patients. Additionally, frequent interventions add to the cost of the therapy. In real-world scenarios, therefore, following recommendations of time-bound endoscopy do not seem practical. There is hence a need to rethink and reposition endoscopy, performed for assessment for mucosal healing, in the management algorithm for patients with UC.
Conventionally, evaluation of clinical symptoms and endoscopic assessment have been the 2 parameters to monitor disease activity in patients with UC. Resolution of clinical symptoms (increased frequency of stools, urgency and rectal bleeding) is the first therapeutic target. A parallelism between clinical symptoms and endoscopic disease activity has been described [9,10]. Partial Mayo Clinic score (including only the clinical components of disease activity) correlates well with the total Mayo Clinic score (including both clinical and endoscopic components of disease activity) [11,12]. A careful assessment of the clinical symptoms can, therefore, correlate with the severity of inflammatory endoscopic lesions. However, discordance between clinical symptoms and disease activity may exist. Even with complete abatement of symptoms (i.e., symptomatic remission), there could be endoscopically and histologically active disease [13-15]. Nearly half of the patients in symptomatic remission have been reported to have evidence of active disease on endoscopy [16-18]. Conversely, a proportion of patients with endoscopic mucosal healing may have persistent symptoms due to superimposed irritable bowel syndrome (IBS) [19,20]. Nevertheless, it is important to rule out active inflammation in such patients who have supposedly functional symptoms.
The disagreement between clinical symptoms and degree of inflammation in a subgroup of patients led to the development of noninvasive biomarkers of inflammation such as erythrocyte sedimentation rate, C-reactive protein, and FC. Of these, FC has been demonstrated to correlate with clinical disease activity (partial Mayo Clinic score) and endoscopic disease activity (endoscopic Mayo Clinic score and Ulcerative Colitis Endoscopic Index of Severity) [21]. FC also has moderate correlation with the histological indices of disease activity [14,16,17,22]. It has been hypothesized that endoscopic and histological resolution of inflammation will result in reduction in the amount of neutrophil migration into the gut lumen and therefore low FC values [23,24]. Thus, FC appears to be a reliable marker to monitor treatment response in UC.
Various concentration thresholds of FC have been proposed across correlation studies to predict mucosal healing. The FC cutoff values depend upon the commercial kit used, type of assay and the population to which the test is applied. Theede et al. [16] demonstrated that a cutoff level of FC of 192 μg/g correlated with endoscopic evidence of mucosal healing, while a cutoff of 171 μg/g identified patients with histologic evidence of mucosal healing. FC values < 150 μg/g have been shown to correlate with endoscopic Mayo Clinic score of 0 [18]. In another retrospective analysis, an FC level of ≤ 60 μg/g predicted endoscopic Mayo Clinic score 0/1 and Nancy score ≤ 1 [25]. A systematic review and meta-analysis proposed a cutoff of ≤ 50 μg/g to be predictive of mucosal (both endoscopic and histologic) healing [22]. On the other hand, FC > 250 μg/g was associated with mucosal/histologic activity in a majority of the patients [26]. Basis the existing literature, FC values less than 50–150 μg/g in patients with UC may suggest endoscopic (endoscopic Mayo Clinic score 0 or 1) and/or histological mucosal healing; though a single cutoff value to discriminate between active and inactive UC, using endoscopy as the reference, has not been validated as yet. Performing colonoscopy in patients with high FC (> 150 μg/g), at the suggested 3–6 months’ time frame, is unlikely to reveal mucosal healing, which, as a matter of fact, is the very purpose of doing colonoscopy.
FC can also identify patients in clinical remission who are at risk for an impending relapse. A high FC (> 50–150 μg/g) in patients in clinical remission correlates with an increased probability of relapse over the next 2–3 months [27-31]. It has been reported that patients who relapse, have high FC values 4–6 months before the apparent clinical relapse. Consecutive normal FC values, on the other hand, are associated with a high probability of maintaining remission over the next couple of months [28,32]. Additionally, FC values at 3 months after initiation of therapy in patients with new-onset UC have been documented to predict the subsequent disease course [33]. Serial testing of FC can hence aid in therapeutic decision making regarding timing of the endoscopy. Two consecutive normal FC values, done at an interval of 2–3 months, are likely to correlate with absence of inflammatory activity on endoscopy.
The FC values can also differentiate between IBS and inflammatory bowel disease. In patients with symptoms attributed to IBS, a normal FC would suggest against endoscopic activity and prevent an unwanted endoscopy.
As is evident, evaluation of clinical symptoms and inflammatory biomarkers can provide adequate information about disease activity. Performing endoscopy upfront, for assessment of mucosal healing in all patients, therefore, is not an obligation and the decision to perform endoscopy has to be individualized based on the response to therapy.
An endoscopic assessment performed too early or delayed for long would not meet the objective(s) of performing endoscopy. As is evident in various induction trials in UC, early endoscopic assessment (within 12–16 weeks) yields mucosal healing rates varying between 25% and 50% only [34-39]. Similarly, delaying endoscopy for too long may result in suboptimal therapy. Therefore, it is important to perform endoscopy for mucosal assessment at the right time.
An important determinant of the right time to perform endoscopic assessment in UC is the “time to therapeutic response” for a particular therapeutic agent. The initial response to therapy is resolution of clinical symptoms (rectal bleeding, frequent stools, and urgency). This generally occurs as early as 3–5 days with intravenous corticosteroids, takes around 2–8 weeks with most of the other therapeutic agents (5-aminosalicylates, oral corticosteroids, infliximab, adalimumab, and golimumab), and may even be prolonged up to 10–20 weeks, as with certolizumab and vedolizumab [40,41]. Immunomodulators like thiopurines might take up to 3–4 months for reaching their maximal therapeutic efficacy, and endoscopic resolution may take even longer than that. Therefore, repeating endoscopy before the expected time to therapeutic response may not be rational. With the availability of sensitive noninvasive inflammatory biomarkers like FC that correlate with the clinical, endoscopic and histological disease activity, the timing of endoscopic assessment, for assessment of mucosal healing, can be guided by serial FC values, in combination with the clinical symptoms.
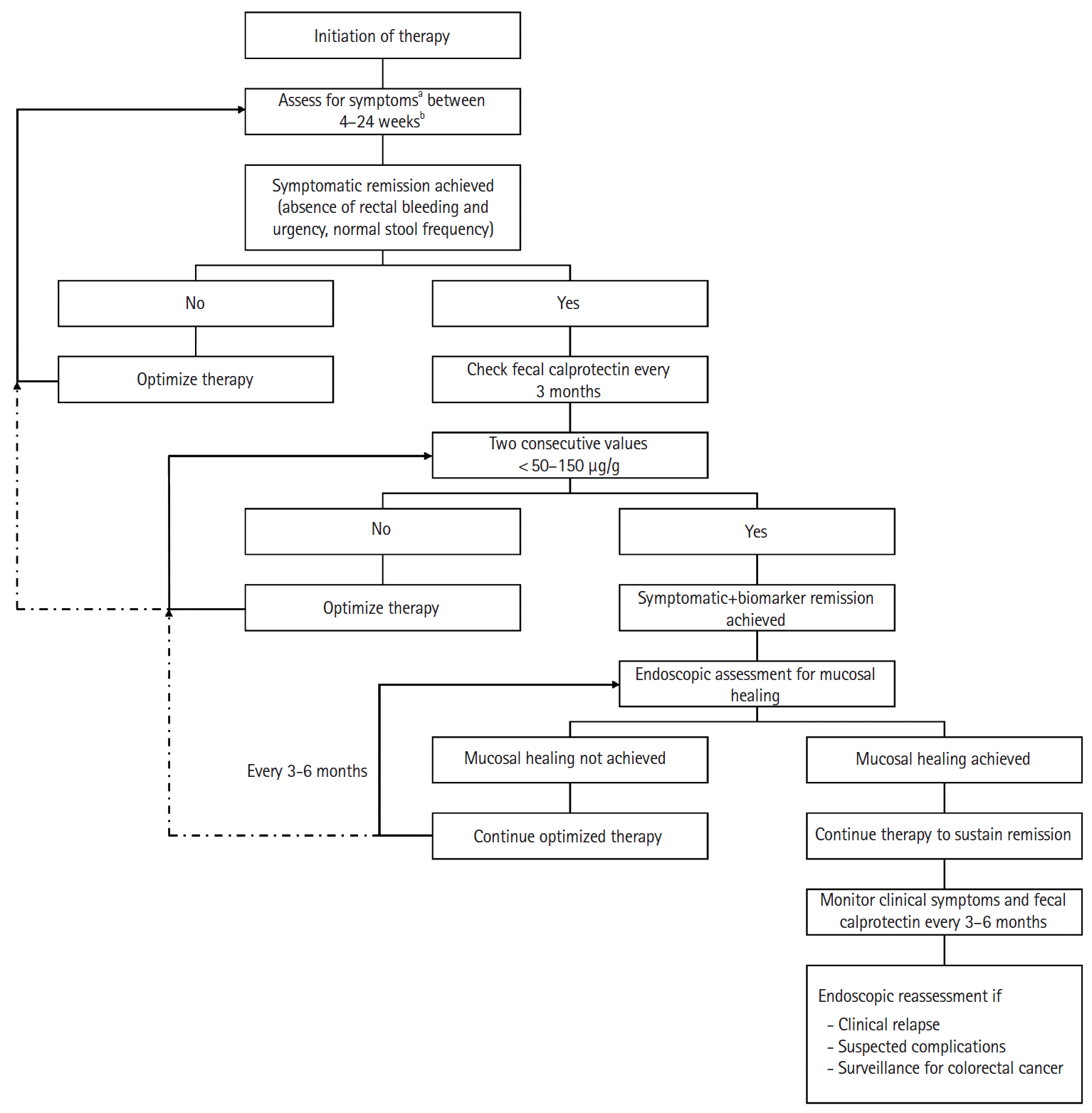
In light of the published evidence, it may be put forward that patients who achieve symptomatic remission should be tested for biomarker remission using FC every 3 months, and when 2 consecutive values of FC are < 50–150μg/g, endoscopy should be considered to look for mucosal healing. If mucosal healing is achieved, the therapeutic agent is continued to sustain remission and further endoscopic assessments are not needed, unless there is clinical relapse (worsening/new symptoms and elevated FC), suspected complications or need for surveillance for colorectal cancer. Patients who do not achieve endoscopic mucosal healing should be evaluated for optimization of therapy (Fig. 1).
In the authors’ opinion, the indications of performing endoscopy in patients with active disease include evaluation of reasons for nonresponse; for example, infections like cytomegalovirus, Epstein-Barr virus, etc., misdiagnosis of Crohn’s disease as UC, presence of colorectal cancer or true drug resistance.
As majority of the patients with UC have rectal involvement and the maximal disease severity is seen in the distal colon, a limited unprepared sigmoidoscopy can suffice. However, in patients with persistent symptoms and a normal/near normal distal colon, full length colonoscopy should be performed.
The appropriate intervals for endoscopic assessment of mucosal healing after initiation of therapy are not clearly defined. “Time bound” endoscopic assessment of all patients every 3–6 months, as recommended by STRIDE/ECCO-ESGAR guidelines, may neither be practical nor useful in real-world scenarios. Frequent endoscopies are also likely to encounter resistance from patients. Endoscopic assessments for mucosal healing should, therefore, be considered once symptomatic and biomarker remissions have been achieved (with the aim to document mucosal healing), rather than as a predetermined time-bound drill. We must not allow the clock and the calendar to blind us to the fact that decision about the timing of endoscopy needs to be individualized. A “response guided” stepwise approach of attaining symptomatic remission, biomarker remission and then evaluating for endoscopic (and histological) remission may be more rational, cost-effective and patient-friendly.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
REFERENCES
1. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019; 13:144–164.

2. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019; 114:384–413.

3. American Society for Gastrointestinal Endoscopy Standards of Practice Committee, Shergill AK, Lightdale JR, et al. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc. 2015; 81:1101–1121.

4. Darr U, Khan N. Treat to target in inflammatory bowel disease: an updated review of literature. Curr Treat Options Gastroenterol. 2017; 15:116–125.

5. Peyrin-Biroulet L, Ferrante M, Magro F, et al. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. J Crohns Colitis. 2011; 5:477–483.

6. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010; 138:463–468.

7. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015; 110:1324–1338.
8. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583.

9. Babić E, Bevanda M, Karin M, et al. Correlation of clinical and endoscopic indices in IBD patients in University Clinical Hospital Mostar. Psychiatr Danub. 2016; 28 Suppl 2:242–246.
10. Kim DB, Lee KM, Lee JM, et al. Correlation between histological activity and endoscopic, clinical, and serologic activities in patients with ulcerative colitis. Gastroenterol Res Pract. 2016; 2016:5832051.

11. Naegeli AN, Hunter T, Dong Y, et al. Full, partial, and modified permutations of the Mayo score: characterizing clinical and patient-reported outcomes in ulcerative colitis patients. Crohns Colitis 360. 2021; 3–otab007.

12. Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008; 14:1660–1666.

13. Rosenberg L, Lawlor GO, Zenlea T, et al. Predictors of endoscopic inflammation in patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis. 2013; 19:779–784.

14. Magro F, Lopes J, Borralho P, et al. Comparison of different histological indexes in the assessment of UC activity and their accuracy regarding endoscopic outcomes and faecal calprotectin levels. Gut. 2019; 68:594–603.

15. Theede K, Holck S, Ibsen P, Kallemose T, Nordgaard-Lassen I, Nielsen AM. Fecal calprotectin predicts relapse and histological mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2016; 22:1042–1048.

16. Theede K, Holck S, Ibsen P, Ladelund S, Nordgaard-Lassen I, Nielsen AM. Level of fecal calprotectin correlates with endoscopic and histologic inflammation and identifies patients with mucosal healing in ulcerative colitis. Clin Gastroenterol Hepatol. 2015; 13:1929–1936.

17. Zittan E, Kelly OB, Kirsch R, et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn’s disease. Inflamm Bowel Dis. 2016; 22:623–630.

18. Sandborn WJ, Panés J, Zhang H, Yu D, Niezychowski W, Su C. Correlation between concentrations of fecal calprotectin and outcomes of patients with ulcerative colitis in a phase 2 trial. Gastroenterology. 2016; 150:96–102.

19. Szałwińska P, Włodarczyk J, Spinelli A, Fichna J, Włodarczyk M. IBS-symptoms in IBD patients-manifestation of concomitant or different entities. J Clin Med. 2020; 10:31.

20. Hoekman DR, Zeevenhooven J, D’Haens GR, Benninga MA. The prevalence of irritable bowel syndrome-type symptoms in inflammatory bowel disease patients in remission. Eur J Gastroenterol Hepatol. 2017; 29:1086–1090.

21. Lee SH, Kim MJ, Chang K, et al. Fecal calprotectin predicts complete mucosal healing and better correlates with the ulcerative colitis endoscopic index of severity than with the Mayo endoscopic subscore in patients with ulcerative colitis. BMC Gastroenterol. 2017; 17:110.
22. Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015; 110:802–819.

23. Hanai H, Takeuchi K, Iida T, et al. Relationship between fecal calprotectin, intestinal inflammation, and peripheral blood neutrophils in patients with active ulcerative colitis. Dig Dis Sci. 2004; 49:1438–1443.

24. De Vos M, Dewit O, D’Haens G, et al. Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naïve patients with ulcerative colitis. J Crohns Colitis. 2012; 6:557–562.

25. Patel A, Panchal H, Dubinsky MC. Fecal calprotectin levels predict histological healing in ulcerative colitis. Inflamm Bowel Dis. 2017; 23:1600–1604.

26. Urushikubo J, Yanai S, Nakamura S, et al. Practical fecal calprotectin cut-off value for Japanese patients with ulcerative colitis. World J Gastroenterol. 2018; 24:4384–4392.

27. Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005; 54:364–368.

28. Heida A, Park KT, van Rheenen PF. Clinical utility of fecal calprotectin monitoring in asymptomatic patients with inflammatory bowel disease: a systematic review and practical guide. Inflamm Bowel Dis. 2017; 23:894–902.

29. D’Incà R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008; 103:2007–2014.

30. Ferreiro-Iglesias R, Barreiro-de Acosta M, Otero Santiago M, et al. Fecal calprotectin as predictor of relapse in patients with inflammatory bowel disease under maintenance infliximab therapy. J Clin Gastroenterol. 2016; 50:147–151.

31. Garcia-Planella E, Mañosa M, Chaparro M, et al. Serial semiquantitative measurement of fecal calprotectin in patients with ulcerative colitis in remission. Scand J Gastroenterol. 2018; 53:152–157.

32. Yamamoto T, Shimoyama T, Matsumoto K. Consecutive monitoring of faecal calprotectin during mesalazine suppository therapy for active rectal inflammation in ulcerative colitis. Aliment Pharmacol Ther. 2015; 42:549–558.

33. Lasson A, Simrén M, Stotzer PO, Isaksson S, Ohman L, Strid H. Fecal calprotectin levels predict the clinical course in patients with new onset of ulcerative colitis. Inflamm Bowel Dis. 2013; 19:576–581.

34. Sandborn WJ, Regula J, Feagan BG, et al. Delayed-release oral mesalamine 4.8 g/day (800-mg tablet) is effective for patients with moderately active ulcerative colitis. Gastroenterology. 2009; 137:1934–1943.

35. Lichtenstein GR, Ramsey D, Rubin DT. Randomised clinical trial: delayed-release oral mesalazine 4.8 g/day vs. 2.4 g/day in endoscopic mucosal healing: ASCEND I and II combined analysis. Aliment Pharmacol Ther. 2011; 33:672–678.

36. Gross V, Bar-Meir S, Lavy A, et al. Budesonide foam versus budesonide enema in active ulcerative proctitis and proctosigmoiditis. Aliment Pharmacol Ther. 2006; 23:303–312.

37. Ardizzone S, Maconi G, Russo A, Imbesi V, Colombo E, Bianchi Porro G. Randomised controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut. 2006; 55:47–53.

38. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476.

39. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012; 142:257–265.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download