Abstract
Background/Aims
Methods
Results
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contribution
Conceptualization: Sharma V. Data curation: Singh AK, Jena A, Jha DK. Formal analysis: Kumar-M P. Methodology: Singh AK. Supervision: Sharma V. Validation: Sharma V. Writing - original draft: Singh AK, Jena A, Kumar-M P, Jha DK. Writing - review & editing: Sharma V. Approval of final manuscript: all authors.
Supplementary Material
REFERENCES
Fig. 1.
Fig. 2.
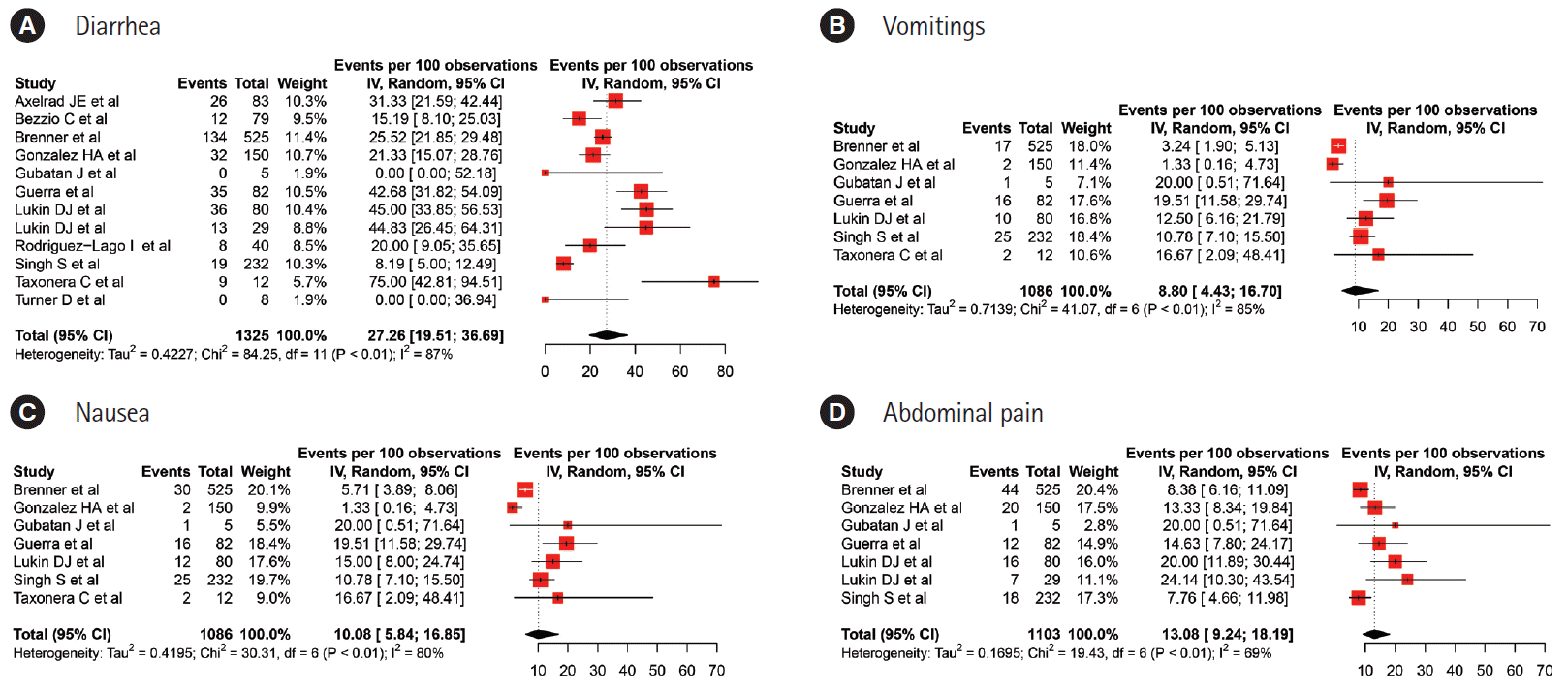
Fig. 3.
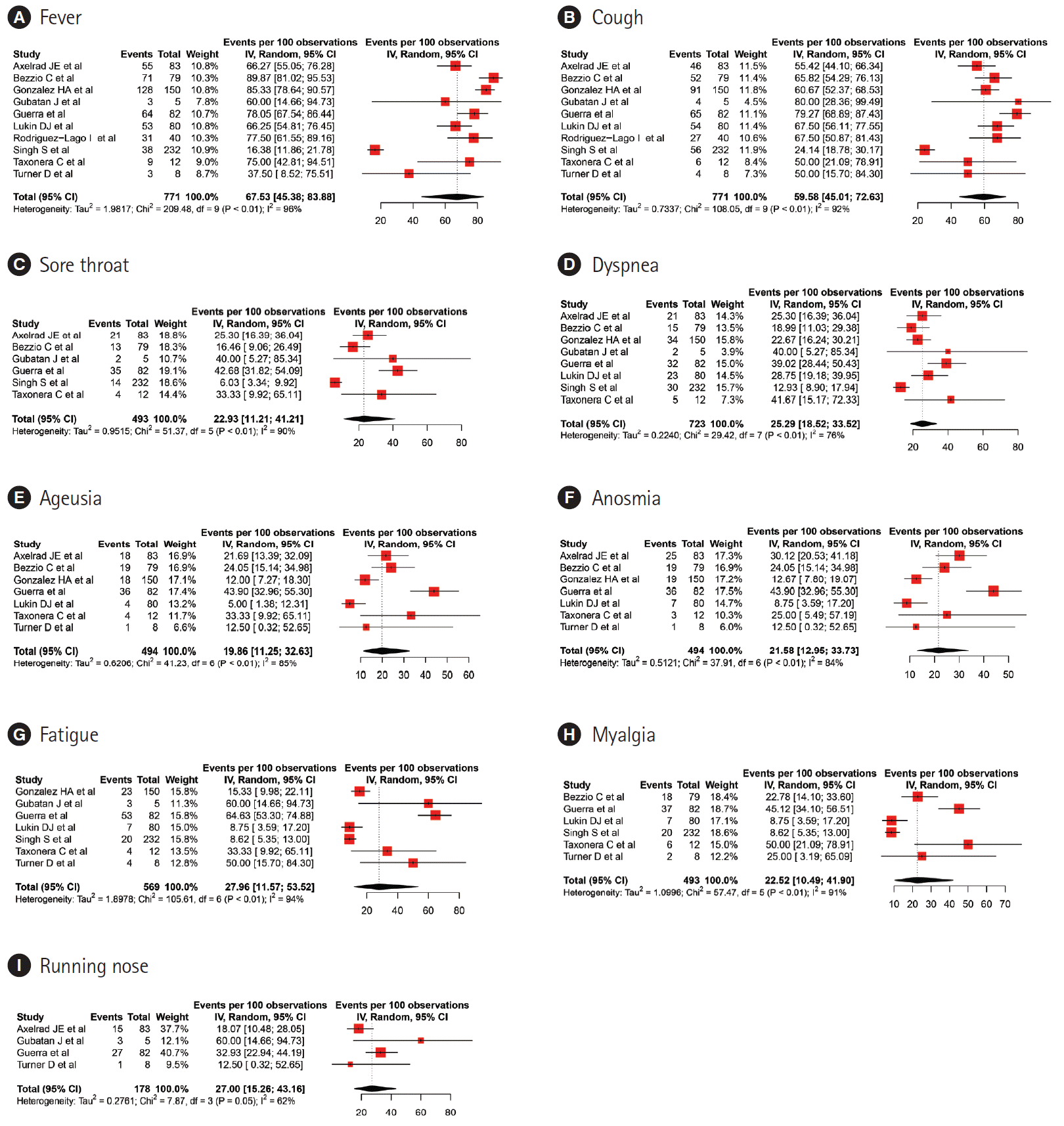
Fig. 4.
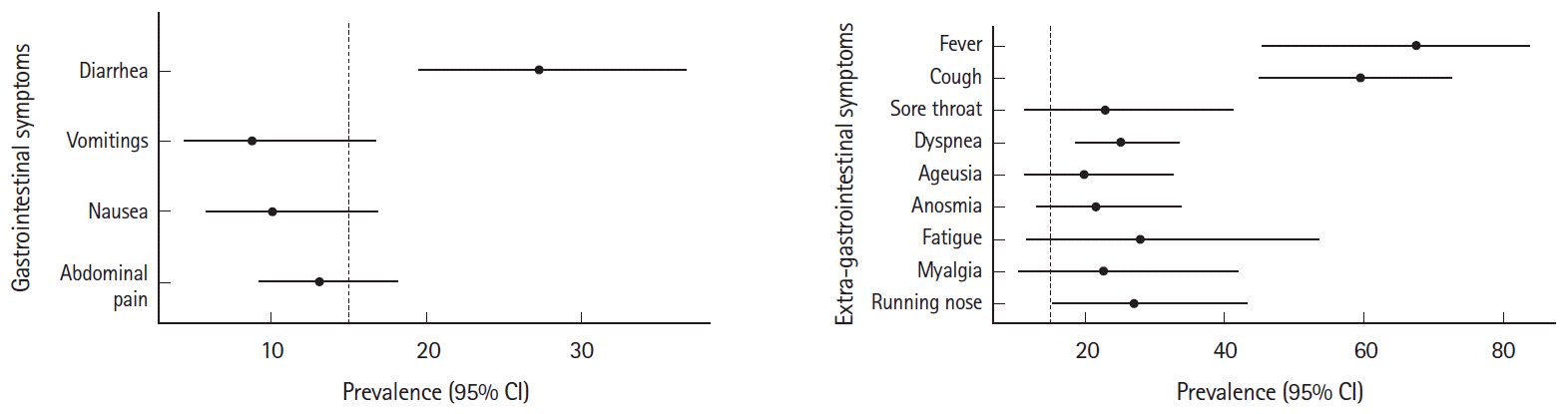
Table 1.
| Study | Country | No. of total IBD | No. of COVID-19 IBD | Ratio of M:F | Age (yr) | UC and CD | IBD medications at the time of COVID-19 diagnosis |
|---|---|---|---|---|---|---|---|
| Axelrad et al. [5] | USA | NA | 83 (confirmed or highly suspected) | 1.13:1 | 35 (27–45) | UC-27, CD-56 | ASA-13, steroid-10, IMM-6, biologics-58, anti-TNF-44 |
| Brenner et al. [4] | Multicenter, multinational | NA | 525 (RT-PCR) | 1.13:1a | 42.9 ± 18.2 | UC-210, CD-312, data missing-3 | ASA-117, steroids-55, IMM-58, biologics-333, JAK-inhibitors-8, others-22 |
| Bezzio et al. [3] | Italy | NA | 79 (RT-PCR in 49 and clinical suspicion plus radiology in 30) | 1.26:1 | 45 (18–80) | UC-47, CD-32 | ASA-24, steroid-9, IMM-7, biologics-47, anti- TNF-29 |
| Gonzalez HAb | Multicenter (Italy, Spain,UK) | NA | 150 (RT-PCR or clinical suspicion) | NA | NA | UC-67, CD-83 | ASA-41, steroid-12, IMM-71, biologics-96, anti-TNF-39 |
| Gubatan et al. [10] | USA | 168 | 5 (RT-PCR) | 1:1.5 | 70.6 ± 4.2 | UC-3, CD-2 | ASA-4, steroid-1, IMM-1, biologics-1, anti-TNF-1 |
| Guerra et al. [11] | Spain | 923 | 82 (RT-PCR in 28 or high clinical suspicion in 54) | 1:1.1 | 46 ± 14 | UC-40, CD-42 | ASA-41, steroid-0, IMM-29, biologics-20, anti-TNF-17 |
| Lukin et al. [12] | USA | 119 | 29 (RT-PCR or high clinical suspicion) | 1:1.42 | NA | UC-14, CD-15 | ASA-11, steroid-19, IMM-2, biologics-21 |
| Lukin et al. [12] | USA | NA | 80 (RT-PCR or high clinical suspicion) | 1.28:1 | 48.3 ± 18.3 | NA | NA |
| Rodríguez-Lago et al. [13] | Spain | NA | 40 (RT-PCR based) | 1.5:1 | 59 (48–68) | UC-27, CD-13 | ASA-26, steroid-4, IMM-13, biologics-9, anti-TNF-4 |
| Singh et al. [14] | USA | 196,403 | 232 (RT-PCR or ICD code based) | NA | 51.2 ± 18.1 | UC-131, CD-101 | NA |
| Taxonera et al. [15] | Spain | 1,918 | 12 (RT-PCR) | 1:3 | 52 ± 16 | UC-5, CD-7 | ASA-4, ateroid-0, IMM-6, biologics-5, anti- |
| Turner et al. [16] | Multicenter, multinational | NA | 8 (RT-PCR or clinical suspicion) | 1.67:1 | NA | UC-3, CD-5 | ASA-4, steroid-1, IMM-4, biologics-5 |
IBD, inflammatory bowel disease; COVID-19, coronavirus disease 2019; M, male; F, female; UC, ulcerative colitis; CD, Crohn’s disease; ASA, Aminosalicylate; IMM, Immunomodulator; TNF, tumor necrosis factor; RT-PCR, reverse transcriptase polymerase chain reaction; JAK, Janus Kinase; ICD, 10th revision of the International Statistical Classification of Diseases and Related Health Problems; NA, not available.
Table 2.
| Study | No. of patients |
Non-GI manifestations, % (95% CI) |
GI manifestations, % (95% CI) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever | Cough | Fatigue | Dyspnea | Myalgia | Sore throat | Running nose | Diarrhea | Nausea/vomiting | Loss of appetite | Pain abdomen | |||
| Non-IBD population | |||||||||||||
| Mao et al. [23] | 6,686 | - | - | - | - | - | - | - | 9 (6–12) | 6 (5–9) | 21 (9–44) | 3 (2–5) | |
| Cheung et al. [24] | 4,243 | - | - | - | - | - | - | - | 12.5 (9.6–16) | 10.2 (6.6–15.3) | 26.8 (16.2–40.8) | 9.2 (5.7–14.5) | |
| Li et al. [25] | 1,994 | 88.5 | 68.6 | 35.8 | 21.2 | - | - | - | 4.8 | 3.9 | - | - | |
| Suresh Kumar et al. [26] | 2,477 | - | - | - | - | - | - | - | 7.8 | 5.5 | 27.1 | 2.7 | |
| Parasa et al. [27] | 1,484 | - | - | - | - | - | - | - | 7.4 (4.3–12.2) | 4.6 (2.6–8) | - | - | |
| Yang et al. [28] | 1,576 | 91.3 (86–97) | 67.7 (59–76) | 51 (34–68) | 30.4 (21–40) | - | - | - | - | - | - | - | |
| Tariq et al. [29] | 12,797 | - | - | - | - | - | - | - | 12.4 (8.2–17.1) | 9 (5.5–12.9) | 22.3 (11.2–34.6) | 6.2 (2.6–10.3) | |
| Ghayda et al. [30] | 5,196 | 77 (69–85) | 60 (48–71) | 31 (23–40) | 25 (20–31) | - | - | - | 6 (5–8) | 5 (3–6) | - | - | |
| Wang et al. [31] | 3,024 | - | - | - | - | - | - | - | 9.1 (6.3–11.9) | 5.2 (3.5–7) | - | 3.5 (1.7–5.4) | |
| Liu et al. [32] | 3,302 | 85.9 (84.6–87.2) | 60.5 (58.8–62.3) | 32.2 (30.5–33.9) | - | - | - | - | - | - | - | - | |
| Kumar et al. [33] | 8,301 | 83 | 61 | - | 18 | - | - | 9 | 5 | - | 4 | ||
| Borges do Nascimento et al. [34] | 91,621 | 84 (80–87) | - | - | 6 (2–11) | - | - | - | - | - | - | - | |
| Qiu et al. [35] | 2,401 | 70.6–100 | 22.4–78 | 22–61.9 | 38.89–85.7 | - | - | - | - | - | - | - | |
| Pormohammad et al. [36] | 61,742 | 87 (65–91) | 68 (55.5–74) | - | - | - | - | - | |||||
| Zhu et al. [37] | 8,697 | 78.4 | 58.3 | 34 | 20.6 | 21.9 | 11.6 | 7.3 | 8.2 | 6.6 | 22.9 | - | |
| IBD population | |||||||||||||
| Current study | 1,325 | 67.53 (45.38–83.88) | 59.58 (45.01–72.63) | 27.96 (11.57–53.52) | 25.29 (18.52–33.52) | 22.52 (10.49–41.9) | 22.39 (11.21–41.21) | 27 (15.26–43.16) | 27.26 (19.51–36.69) | 10.08 (5.84–16.85)/8.8(4.43–16.7) | - | 13.08 (9.24–18.19) | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download