METHODS
1. Study Design
This prospective open-label single arm cohort study was conducted in the Department of Gastroenterology and Hepatobiliary Sciences at Fortis Memorial Research Institute, a quaternary care hospital in the National Capital Region of India. Recruitment commenced in November 2014. The study was approved by the Institutional Ethics Committee (vide numbers: 2014-004IP-11 dated October 22, 2014, and 2016-001IP-15 dated February 8, 2015). Written informed consent was obtained from all subjects.
2. Study Population
Patients, 18 years or older, with active UC (Mayo score 4 to 10 with endoscopic subscore ≥ 1) of more than 1-year duration, who were corticosteroid dependent (defined as inability to reduce steroids below prednisolone 10 mg/day within 3 months of starting steroids or relapse within 3 months of stopping steroids) despite use of azathioprine 2 mg/kg or were thiopurine intolerant, were offered FMT in addition to standard of care.
The diagnosis of UC was established according to the Lennard-Jones criteria [
7]. Stable maintenance dose of medication for 4 weeks for oral corticosteroids (maximum dose of prednisolone 20 mg daily) and oral/rectal 5-aminosalicylates and 8 weeks for azathioprine was required for inclusion. Use of biologicals, antibiotics or probiotics was not allowed for 8 weeks prior to recruitment. Recipient evaluation included complete blood count, C-reactive protein, serum albumin and colonoscopy with biopsy and immunohistochemistry for cytomegalovirus. Stool tests included ova and cysts, culture, modified Ziehl-Neelsen stain,
Cryptosporidium antigen and
C. difficile toxin A and B. Fecal calprotectin, once available, was done as required. Patients were excluded if they had ulcerative proctitis confined to distal 5 cm, indeterminate colitis, previous colonic surgery, irritable bowel syndrome, pregnancy, significant comorbidity or severe UC defined by need for hospitalization, Mayo score of more than 10 or by the Truelove and Witts criteria.
3. Intervention
Volunteer stool donors, arranged by the patients, were prospectively screened and tested as per European consensus on FMT in clinical practice to rule out communicable diseases, gastrointestinal, rheumatic, allergic and metabolic disorders [
8]. Stool donors who received antibiotics within the preceding 3 months were excluded. Fresh donor stool was collected and prepared at home for FMT. Two hundred grams of stool was measured in an ice cream cup, blended with 300 mL saline, filtered, drawn up into 7 syringes of 50 mL each and transported to hospital on ice. The stool was allowed to stand till the temperature was above 30°C and instilled within 6 hours of collection. Bowel preparation for colonoscopy was done with polyethylene glycol lavage, a night before the procedure. Ileo-colonoscopy was performed under conscious sedation and prepared stool instilled into the terminal ileum, caecum, ascending colon, transverse colon and proximal descending. Rectal biopsy specimens were collected. The recipient was kept nil by mouth for 4 hours and administered intravenous fluids and tablet loperamide 4 mg 30 minutes and 4 hours following FMT. Patients were asked to report the time of passage of first stool following FMT. Two more sessions of colonoscopic induction FMT (iFMT) were performed at fortnightly intervals. Thereafter, patients were asked to report every 4 weeks for clinical evaluation and sigmoidoscopy with biopsy for 24 weeks.
4. Definitions, Drug Tapering, Maintenance, and Rescue FMT
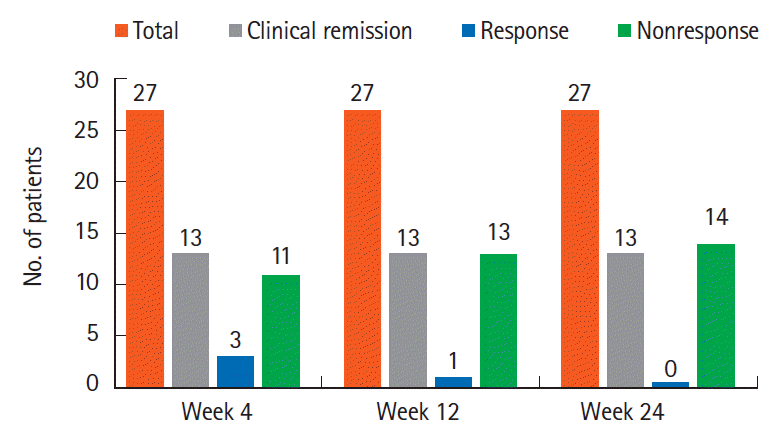
Clinical remission (CR) was defined as Mayo score ≤ 2 with endoscopic subscore ≤ 1 and clinical response as reduction in Mayo score by ≥ 3 over baseline. In patients who achieved CR or response at 4 weeks, prednisolone was tapered by 5 mg weekly. If CR or clinical response were maintained at 12 weeks following corticosteroid withdrawal, azathioprine was tapered by 25 mg weekly.
Long-term patients were maintained only on oral 5-aminosalicylates and advised to undergo maintenance FMT (mFMT) every 6 months, defined as FMT done for asymptomatic patients with Mayo score 0 to 3 and endoscopy subscore 0. Those who developed recurrence of symptoms and developed relapse were offered rescue FMT (rFMT), defined as FMT for symptomatic patients with Mayo score 4-10 and endoscopy subscore ≥ 1. If there was no improvement in 2 weeks, low dose prednisolone, 20 mg daily, was added for 4 weeks and tapered over next 4 weeks. Patients who did not achieve CR or clinical response were offered FMT from a different stool donor.
Rectal biopsies specimens were evaluated and graded using Geboes grading system [
9], by a histopathologist with 19 years of experience, who was blinded to the clinical data. Parameters studied included architectural change, chronic inflammatory infiltrate, increase in eosinophils and neutrophils in lamina propria, cryptitis, crypt distortion, erosion, and ulceration. The Geboes score ranges from 0 to 5.4 and UC was considered active when score was ≥ 3.1. Geboes score less than 3.1 was defined as histological response.
5. Outcome Measures
Primary outcome was corticosteroid-free CR or clinical response 12 weeks following FMT. Secondary outcomes were corticosteroid-free CR and clinical response at 24 weeks, corticosteroid- and azathioprine-free CR and response at 24 weeks, ability to maintain remission with mFMT and efficacy of rFMT.
6. Statistical Methods
Descriptive analysis was carried out. Categorical variables were presented in number and percentage (%) and continuous variables were presented as mean ± standard deviation or median (range). Comparisons between responder and nonresponder groups were performed by applying chi-square statistic or Fisher exact test for categorical data and Student t-test (unpaired) for normally distributed continuous variables. Comparisons of nonparametric data between groups were performed with the Mann-Whitney U-test. Wilcoxon signed-rank test was used to compare average Mayo scores before and after FMT. Statistical significance is assumed at a value of P=0.05. All statistical analyses were performed with SPSS statistics software, version 22.0 (IBM Corp., Armonk, NY, USA).
Go to :

DISCUSSION
This is the first study on FMT in a real-world setting with a follow-up of up to 5 years. Five randomized clinical trials (RCTs) studied the efficacy of FMT in achieving steroid-free remission at 7 to 12 weeks and another RCT studied the efficacy of FMT in maintaining remission (
Table 4). Four studies that used the colonoscopic or rectal route for iFMT showed benefit over placebo, while the trial with nasojejunal route did not show benefit. Remission rate in the treatment arm varied from 24% to 32% and response rate 29% to 55% [
10-
14]. Several systematic reviews and meta-analyses have been published. Costello et al. [
15] reviewed 14 cohort studies and 4 RCTs that used varying protocols. In the meta-analysis of 4 RCTs, CR was achieved in 39 of 140 (28%) patients in the donor FMT groups compared with 13 of 137 (9%) patients in the placebo groups (odds ratio [OR], 3.67; 95% confidence interval [CI], 1.82–7.39;
P<0.01). Clinical response was achieved in 69 of 140 (49%) donor FMT patients compared to 38 of 137 (28%) placebo patients (OR, 2.48; 95% CI, 1.18–5.21;
P=0.02). In cohort studies, 39 of 168 (24%; 95% CI, 11%–40%) achieved CR. A systematic review and meta-analysis by Narula et al. [
16], including 4 RCTs, FMT was associated with higher combined clinical and endoscopic remission compared with placebo (risk ratio of UC not in remission, 0.80; 95% CI, 0.71–0.89) with a number needed to treat of 5 (95% CI, 4–10). In another meta-analysis by Lam et al. [
17], 4 RCTs and 2 cohort studies were included. FMT was more effective than placebo in inducing CR (OR, 3.85; 95% CI, 2.21–6.7;
P<0.001;
I2=0%) and clinical response (OR, 2.75; 95% CI, 1.33–5.67;
P=0.006;
I2=49%), but there was no statistical difference on steroid-free remission (OR, 2.08; 95% CI, 0.41– 10.5;
P=0.37;
I2=69%).
Table 4.
Summary of Randomized Control Trials in Patients with UC
|
Variable |
Author (year)
|
|
Moayyedi et al. (2015) [10] |
Rossen et al. (2015) [11] |
Paramsothy et al. (2017) [12] |
Crothers et al. (2018) [13] |
Costello et al. (2019) [14] |
Sood et al. (2019) [19] |
|
Country |
Canada |
Austria |
Australia |
USA |
Australia |
India |
|
Route and frequency of administration |
Enema weekly × 6 |
Nasoduodenal tube (0 & 3 weeks) |
Colonoscopic × 1+enema 5 day/week × 8 weeks |
Colonoscopic+daily capsules |
Colonoscopic × 1+ enema × 2 over 7 days |
Colonoscpic every 8 weeks |
|
Dose of FMT |
8.3 g (50 mL)/week |
120 g |
187 g/week |
50 g+0.375 g |
100 g/week |
100 g |
|
Donor |
Random (maximum from 1 star donor) |
Random |
Pooled |
High butyrate |
Pooled, anerobic |
Single |
|
Placebo |
Water |
Autologous stool |
Saline+odorant+colorant |
Sham FMT+placebo capsules |
Autologous stool |
Water with food grade color |
|
Patient selection |
Active UC (Mayo score ≥4) with endoscopic subscore ≥1 |
Mild/moderate UC |
Active UC (Mayo score 4-10) with endoscopic subscore ≥1 and physician global assessment ≤2 |
Mayo score 4-10. Pretreated with 7-day antibiotics |
Mayo score 3-10 with endoscopic subscore ≥2 |
In remission following colonoscopic FMT |
|
Definition clinical remission |
Mayo score ≤2 with endoscopic subscore reduction ≥1 |
SCCAI ≤2 + ≥1 point decrease in Mayo endoscopic score |
Steroid free with Mayo score ≤2 with endoscopic subscore reduction ≥1 and all subscores ≤1 |
Mayo score <3 |
Steroid free with Mayo score ≤2 with endoscopic subscore reduction ≤1 |
Steroid free Mayo score ≤2 with all subscores ≤1 |
|
Definition clinical response |
Reduction in Mayo score ≥3 |
SCCAI reduction ≤1.5 |
Reduction in Mayo score ≥3 |
Reduction in Mayo score ≥3 |
Reduction in Mayo score ≥3 |
NA |
|
Primary end point |
Week 7 |
Week 12 |
Week 8 |
Week 12 |
Week 8 +1 year follow up |
Week 48 |
|
No. |
75 |
48 |
81 |
15 |
73 |
61 |
|
Study group/controls |
38/37 |
23/25 |
41/40 |
07 vs. 8 |
38/35 |
31/30 |
|
Remission |
24% vs. 5% (P=0.03) |
30.4% vs. 32% (P=0.1) |
27% vs. 8% (P=0.021) |
29% vs. 13% |
32% vs. 9% (P=0.03) |
87.1% vs. 66.7% (P=0.111) |
|
Response |
39% vs. 24% (P=0.16) |
47.8% vs. 52% (P=NA) |
54% vs. 23% (P=0.004) |
29% vs. 0% |
55% vs. 23% (P=0.007) |
Endoscopic remission 58.1% vs. 26.7% (P=0.026) |
|
Benefit |
Yes |
No |
Yes |
Yes |
Yes |
Yes |
|
Histological improvement |
Yes |
Not studied |
Not studied |
Decreased inflammation 66% vs. 16.6%; remission 50% vs. 0% |
Not studied |
Histological remission 45.2% vs. 16.7% (P=0.033) |
|
Microbiota diversity |
Improved |
Improved |
Improved at 4 weeks and 8 weeks |
Not studied |
|
Not studied |
|
Microbiota composition |
Improved |
Improved |
Fusobacterium associated with lack of remission |
Not studied |
|
Not studied |
|
Severe adverse effects |
3% vs. 2% |
Nil |
2% vs. 1% |
Not mentioned |
3% vs. 2% |
Nil |
|
Mild adverse effects |
Not mentioned |
78.3% vs. 64% (P=0.28) |
78% vs. 83% |
Not mentioned |
|
Not mentioned |

Our rates of CR and clinical response appear to be better than those in the reported RCTs, despite most of our patients being corticosteroid dependent. It has been fairly well established that colonoscopic delivery of FMT yields better results than upper gastrointestinal routes. In a meta-analysis by Tang et al. [
18], in the 5 studies that transplanted feces via the lower gastrointestinal tract, CR rate was better in the FMT group as com pared to placebo, 50.8% versus 31.4% (OR, 2.70; 95% CI, 1.67–4.37;
P<0.0001). In the 2 studies that delivered FMT through upper gastrointestinal tract, the CR rate in FMT group was not better than placebo (30.0% vs. 30.3%: OR, 0.98; 95% CI, 0.33–2.89;
P=0.98). The dose of stool used does not seem to influence outcome, though we used the higher dose of 140 g per session. Our results may also be influenced by the fact that we used 3 colonoscopic sessions for iFMT as compared to the RCTs that used only 1 colonoscopic session followed by rectal enemas. Sood et al. [
19] performed intensive FMT with 7 sessions of colonoscopic FMT on weeks 0, 2, 6, 10, 14, 18, and 22. At week 24, steroid-free CR was achieved in 19 out of 41 patients (46.3%), whereas clinical response and endoscopic remission were achieved in 31 out of 41 patients (75.6%) and 26 out of 41 patients (63.4%), respectively. All patients with clinical response were able to withdraw steroids. However, the drop rate was high at 8 out of 41 (19.5%). Perhaps, using multiple donors instead of 1 per FMT could have improved our results further. In the recent meta-analysis by Tang et al. [
18], in 4 RCTs that used feces from multiple donors, CR rate was higher in FMT group versus placebo (37.9% vs. 17.5%: OR, 2.93; 95% CI, 1.67–5.71;
P=0.0002), while in 2 studies from a single donor CR in FMT group was not better than placebo (76.3% vs. 57.9%: OR, 2.64; 95% CI, 0.87–8.01;
P=0.09). In our experience, with nonresponders, change of donor did not show any additional benefit in rates of CR or clinical response. We planned a deliberate and slow taper of steroids in patients who showed CR or clinical response. It has been shown that there is incremental effect of donor stool on gut flora of the recipient that may be demonstrable as early 1 week after FMT but it may take 3 months for the biodiversity to match that of the donor [
20]. We feel that too rapid a taper from the day after first FMT, as described by Moayyedi et al. [
10] and Sood et al. [
21], may not be recommended in day-to-day practice.
As seen in most of the large trials, we did not encounter any significant adverse effects over a 5-year period in 109 FMT procedures for UC. Worsening of Mayo score at 4 weeks was seen in 1 of 27 patients (3.7%). Flare of UC following FMT has been reported in 4.6% (95% CI, 1.8%–11%) patients from the RCTs [
22].
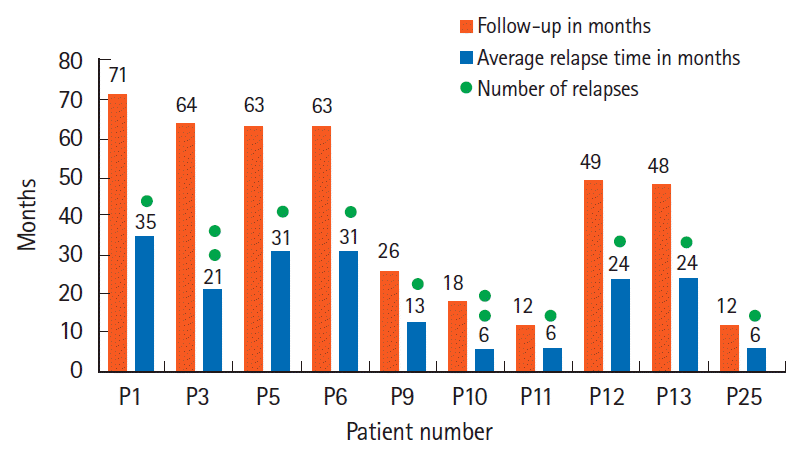
It has been reasonably well established that colonoscopic FMT is more effective than placebo in inducing remission in mild or moderate UC, but the question of how long the effect lasts has not been suitably addressed. Relapse is inevitable. We have shown that patients who opted for mFMT every 6 months had a trend towards maintaining remission for a longer period than those who chose otherwise. Sood et al. [
21], carried out colonoscopic mFMT every 8 weeks for 48 weeks. In the treatment arm, 87% patients maintained remission as compared to 66.7% in the placebo arm (
P=0.111). This regimen appears too intensive as it may be difficult to convince patients to undergo colonoscopic FMT every 8 weeks for a lifetime. We noted that “FMT fatigue” sets in and there is reluctance for mFMT even in patients who are doing well. We have also demonstrated that rFMT by itself is able to induce CR or clinical response in 40% of patients. Another 50% showed response when low-dose corticosteroids were added to rFMT. Suggested algorithms showing possible role of iFMT, mFMT and rFMT in the treatment of patients of CDUC with or without thiopurines is shown in
Figs 4 and
5.
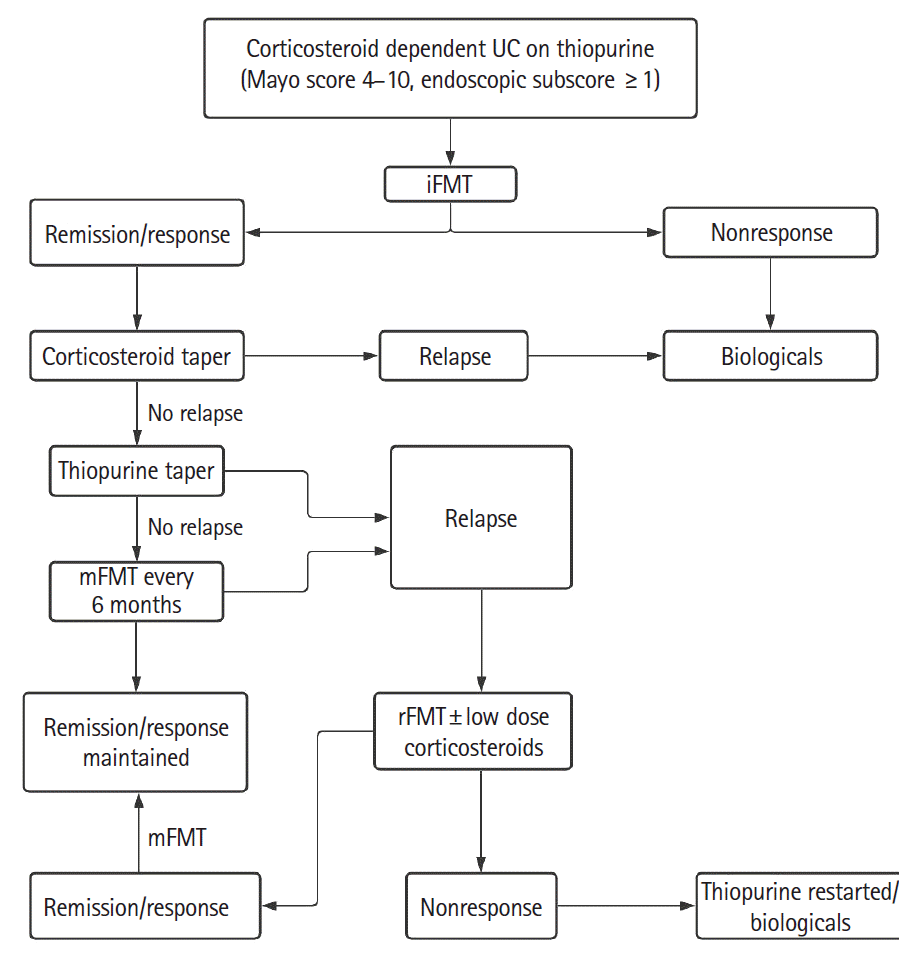 | Fig. 4.Algorithm showing possible role of iFMT, mFMT, and rFMT in the treatment of corticosteroid dependent ulcerative colitis (UC) on thiopurines. FMT, fecal microbiota transplantation; iFMT, induction FMT; mFMT, maintenance FMT; rFMT, rescue FMT. 
|
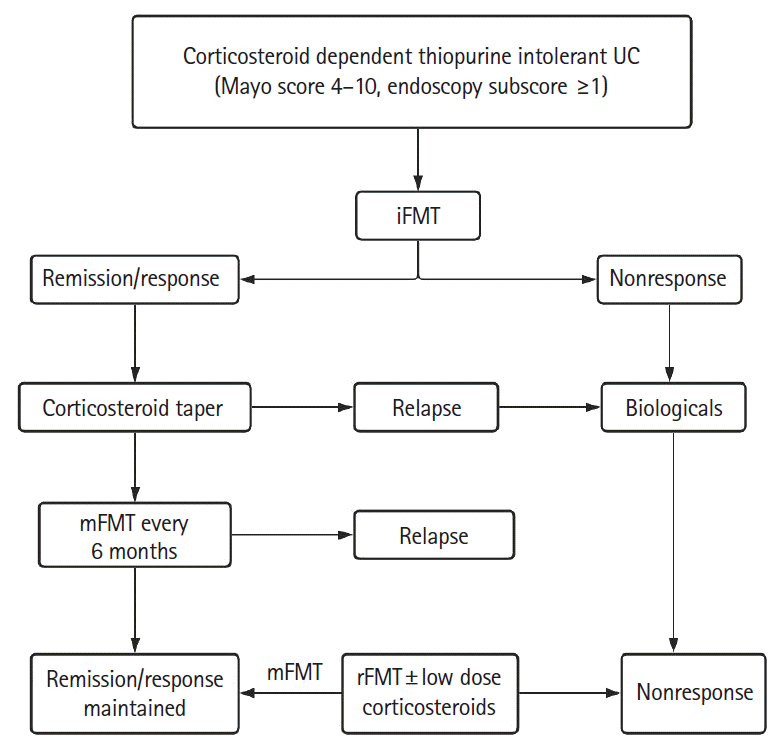 | Fig. 5.Algorithm showing possible role of iFMT, mFMT and rFMT in the treatment of corticosteroid dependent ulcerative colitis (UC) who are thiopurine intolerant. FMT, fecal microbiota transplantation; iFMT, induction FMT; mFMT, maintenance FMT; rFMT, rescue FMT. 
|
This is the first study on FMT for UC where stool preparation was done at home. The kit, including the mixer, cost only Rupees 3,000 (40 USD). Preparation of stool sample at home saved the cost of work space, equipment, salaries and disposables. Contamination was minimized by use of sterile gloves and use of normal saline for rinsing the mixer and utensils and also in the preparation of FMT slurry.
Drawbacks of our study include the absence of a control arm, lack of data on the spectrum of fecal bacteria in recipients and donors and the correlation of CR with improvement in bacterial profile.
FMT is an effective, low cost and safe option for induction of corticosteroid-free and thiopurine-free clinical, endoscopic and histological remission in selected patients with UC. There is a trend towards delay of relapse with 6-month mFMT. For treatment of relapse, rFMT by itself or in association with low dose corticosteroids may be effective when Mayo score is 10 or less. FMT could be offered specially to patients who are corticosteroid dependent or thiopurine intolerant and are candidates for biologicals. Two of our patients who were advised total proctocolectomy but opted for FMT protocol continue to do well and remain corticosteroid-free and thiopurine-free at 6 years and 4 years. Only 1 patient required biologicals during the entire study duration. While the search for the perfect stool for FMT continues, use of stool from star donors or mixed donors may improve the results of FMT for patients with UC.
Go to :





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download