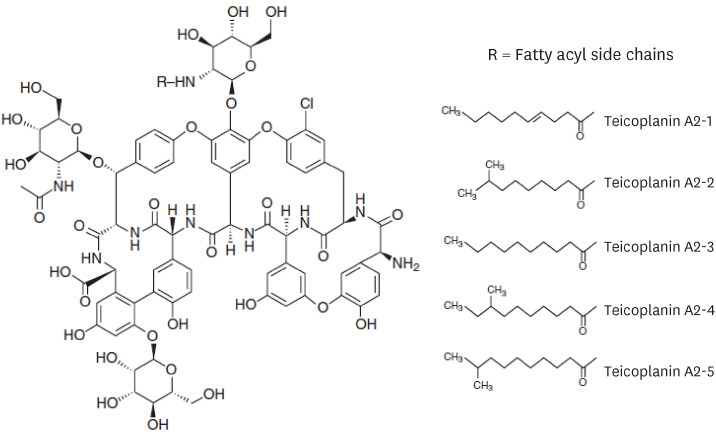
1. Borghi A, Coronelli C, Faniuolo L, Allievi G, Pallanza R, Gallo GG. Teichomycins, new antibiotics from Actinoplanes teichomyceticus nov. sp. IV. Separation and characterization of the components of teichomycin (teicoplanin). J Antibiot (Tokyo). 1984; 37(6):615–620. PMID:
6235204.

2. Bennett J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Amsterdam, Netherlands: Elsevier;2020. p. 387–389.
3. Wilson AP. Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet. 2000; 39(3):167–183. PMID:
11020133.

4. Nakamura A, Takasu O, Sakai Y, Sakamoto T, Yamashita N, Mori S, et al. Development of a teicoplanin loading regimen that rapidly achieves target serum concentrations in critically ill patients with severe infections. J Infect Chemother. 2015; 21(6):449–455. PMID:
25726436.

5. Hanai Y, Takahashi Y, Niwa T, Mayumi T, Hamada Y, Kimura T, et al. Optimal trough concentration of teicoplanin for the treatment of methicillin-resistant
Staphylococcus aureus infection: a systematic review and meta-analysis. J Clin Pharm Ther. 2021; 46(3):622–632. PMID:
33547647.

6. Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant
Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020; 77(11):835–864. PMID:
32191793.

7. Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC Jr, Craig WA, Billeter M, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2009; 29(11):1275–1279. PMID:
19873687.
8. He N, Su S, Ye Z, Du G, He B, Li D, et al. Evidence-based guideline for therapeutic drug monitoring of vancomycin: 2020 update by the division of therapeutic drug monitoring, Chinese Pharmacological Society. Clin Infect Dis. 2020; 71(Suppl 4):S363–S371. PMID:
33367582.

9. Jorgensen SC, Dersch-Mills D, Timberlake K, Stewart JJ, Gin A, Dresser LD, et al. AUCs and 123s: a critical appraisal of vancomycin therapeutic drug monitoring in paediatrics. J Antimicrob Chemother. 2021; 76(9):2237–2251. PMID:
33675656.

10. Kato H, Hagihara M, Okudaira M, Asai N, Koizumi Y, Yamagishi Y, et al. Systematic review and meta-analysis to explore optimal therapeutic range of vancomycin trough level for infected paediatric patients with Gram-positive pathogens to reduce mortality and nephrotoxicity risk. Int J Antimicrob Agents. 2021; 58(2):106393. PMID:
34174409.

11. Lodise TP, Drusano G. Vancomycin area under the curve-guided dosing and monitoring for adult and pediatric patients with suspected or documented serious methicillin-resistant
Staphylococcus aureus infections: putting the safety of our patients first. Clin Infect Dis. 2021; 72(9):1497–1501. PMID:
33740042.

12. Rybak MJ, Le J, Lodise T, Levine D, Bradley J, Liu C, et al. Executive summary: therapeutic monitoring of vancomycin for serious methicillin-resistant
Staphylococcus aureus infections: a revised consensus guideline and review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. J Pediatric Infect Dis Soc. 2020; 9(3):281–284. PMID:
32659787.
13. Smit C, Goulooze SC, Brüggemann RJ, Sherwin CM, Knibbe CA. Dosing recommendations for vancomycin in children and adolescents with varying levels of obesity and renal dysfunction: a population pharmacokinetic study in 1892 children aged 1–18 years. AAPS J. 2021; 23(3):53. PMID:
33839974.
14. Cavalcanti AB, Goncalves AR, Almeida CS, Bugano DD, Silva E. Teicoplanin versus vancomycin for proven or suspected infection. Cochrane Database Syst Rev. 2010; (6):CD007022. PMID:
20556772.
15. Loane G, Gwee A. Teicoplanin therapeutic drug monitoring and treatment outcomes in children with gram-positive infections. J Pediatric Infect Dis Soc. 2021; 10(5):682–685. PMID:
33543753.

16. Svetitsky S, Leibovici L, Paul M. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother. 2009; 53(10):4069–4079. PMID:
19596875.

17. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021; 372(160):n160. PMID:
33781993.

18. Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. London, UK: Cochrane;2022.
20. Kanji S, Hayes M, Ling A, Shamseer L, Chant C, Edwards DJ, et al. Reporting guidelines for clinical pharmacokinetic studies: the ClinPK statement. Clin Pharmacokinet. 2015; 5(7):783–795.

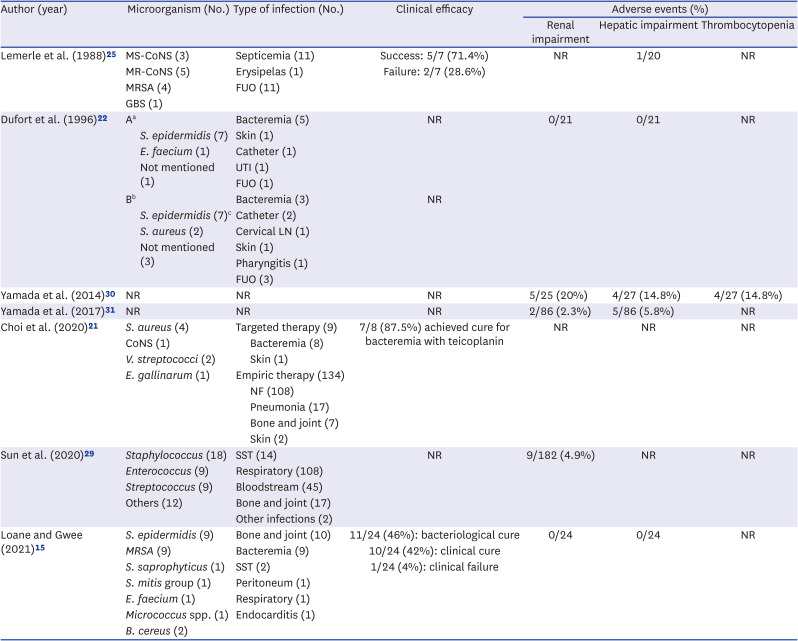
21. Choi JS, Kim JM, Kim D, Kim SH, Cho H, Park HD, et al. Therapeutic drug level monitoring of teicoplanin in Korean pediatric patients with normal versus impaired renal function. J Korean Med Sci. 2020; 35(46):e376. PMID:
33258328.

22. Dufort G, Ventura C, Olivé T, Ortega JJ. Teicoplanin pharmacokinetics in pediatric patients. Pediatr Infect Dis J. 1996; 15(6):494–498. PMID:
8783345.
23. Ito H, Shime N, Kosaka T. Pharmacokinetics of glycopeptide antibiotics in children. J Infect Chemother. 2013; 19(2):352–355. PMID:
22872188.
24. Jung J, Lee K, Oh J, Choi R, Woo HI, Park HD, et al. Therapeutic drug monitoring of teicoplanin using an LC-MS/MS method: analysis of 421 measurements in a naturalistic clinical setting. J Pharm Biomed Anal. 2019; 167:161–165. PMID:
30776754.

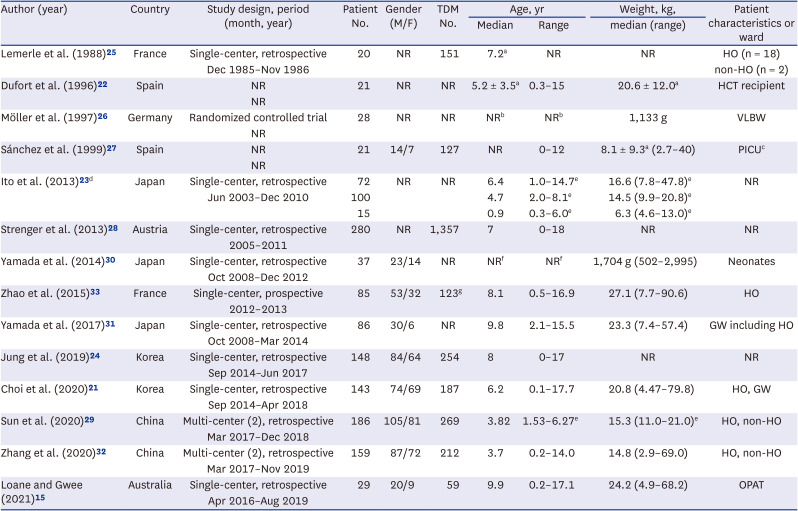
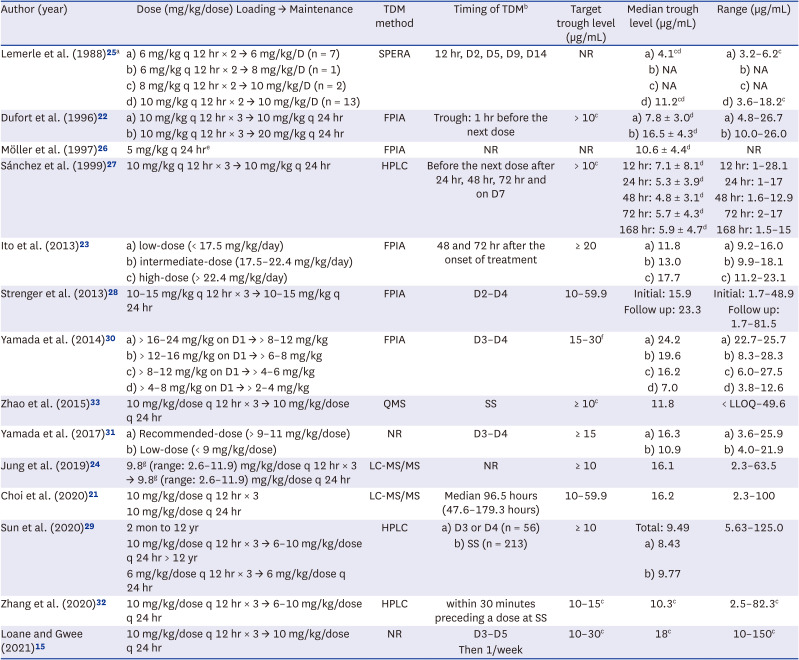
25. Lemerle S, de La Rocque F, Lamy R, Fremaux A, Bernaudin F, Lobut JB, et al. Teicoplanin in combination therapy for febrile episodes in neutropenic and non-neutropenic paediatric patients. J Antimicrob Chemother. 1988; 21:113–116.
26. Möller JC, Nelskamp I, Jensen R, Reiss I, Kohl M, Gatermann S, et al. Comparison of vancomycin and teicoplanin for prophylaxis of sepsis with coagulase negative staphylococci (CONS) in very low birth weight (VLBW) infants. J Perinat Med. 1997; 25(4):361–367. PMID:
9350607.

27. Sánchez A, López-Herce J, Cueto E, Carrillo A, Moral R. Teicoplanin pharmacokinetics in critically ill paediatric patients. J Antimicrob Chemother. 1999; 44(3):407–409. PMID:
10511412.
28. Strenger V, Hofer N, Rödl S, Hönigl M, Raggam R, Seidel MG, et al. Age- and gender-related differences in teicoplanin levels in paediatric patients. J Antimicrob Chemother. 2013; 68(10):2318–2323. PMID:
23702837.

29. Sun D, Zhang T, Mi J, Dong Y, Liu Y, Zhang Y, et al. Therapeutic drug monitoring and nephrotoxicity of teicoplanin therapy in Chinese children: a retrospective study. Infect Drug Resist. 2020; 13:4105–4113. PMID:
33209040.
30. Yamada T, Kubota T, Nakamura M, Ochiai M, Yonezawa M, Yano T, et al. Evaluation of teicoplanin concentrations and safety analysis in neonates. Int J Antimicrob Agents. 2014; 44(5):458–462. PMID:
25218156.
31. Yamada T, Kubota T, Yonezawa M, Nishio H, Kanno S, Yano T, et al. Evaluation of teicoplanin trough values after the recommended loading dose in children with associated safety analysis. Pediatr Infect Dis J. 2017; 36(4):398–400. PMID:
27977550.

32. Zhang T, Sun D, Shu Z, Duan Z, Liu Y, Du Q, et al. Population pharmacokinetics and model-based dosing optimization of teicoplanin in pediatric patients. Front Pharmacol. 2020; 11:594562. PMID:
33363469.

33. Zhao W, Zhang D, Storme T, Baruchel A, Declèves X, Jacqz-Aigrain E. Population pharmacokinetics and dosing optimization of teicoplanin in children with malignant haematological disease. Br J Clin Pharmacol. 2015; 80(5):1197–1207. PMID:
26138279.

34. Kim SW. Therapeutic drug monitoring (TDM) of antimicrobial agents. Infect Chemother. 2008; 40(3):133–139.

35. Hogan PG, Mork RL, Thompson RM, Muenks CE, Boyle MG, Sullivan ML, et al. Environmental methicillin-resistant
Staphylococcus aureus contamination, persistent colonization, and subsequent skin and soft tissue infection. JAMA Pediatr. 2020; 174(6):552–562. PMID:
32227144.

36. Inagaki K, Lucar J, Blackshear C, Hobbs CV. Methicillin-susceptible and methicillin-resistant
Staphylococcus aureus bacteremia: nationwide estimates of 30-day readmission, in-hospital mortality, length of stay, and cost in the United States. Clin Infect Dis. 2019; 69(12):2112–2118. PMID:
30753447.
37. Laupland KB. Incidence of bloodstream infection: a review of population-based studies. Clin Microbiol Infect. 2013; 19(6):492–500. PMID:
23398633.

38. Pereira MR, Rana MM. AST ID Community of Practice. Methicillin-resistant
Staphylococcus aureus in solid organ transplantation-guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019; 33(9):e13611. PMID:
31120612.

39. Guthrie JL, Teatero S, Hirai S, Fortuna A, Rosen D, Mallo GV, et al. Genomic epidemiology of invasive methicillin-resistant
Staphylococcus aureus infections among hospitalized individuals in Ontario, Canada. J Infect Dis. 2020; 222(12):2071–2081. PMID:
32432674.

40. Abebe W, Tegene B, Feleke T, Sharew B. Bacterial bloodstream infections and their antimicrobial susceptibility patterns in children and adults in Ethiopia: a 6-year retrospective study. Clin Lab. 2021; 67(11):

41. Fu P, Xu H, Jing C, Deng J, Wang H, Hua C, et al. bacterial epidemiology and antimicrobial resistance profiles in children reported by the ISPED program in China, 2016 to 2020. Microbiol Spectr. 2021; 9(3):e0028321. PMID:
34730410.

44. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant
Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011; 52(3):285–292. PMID:
21217178.

45. Avedissian SN, Le J, Neely MN, Cortés-Penfield N, Bradley J, Rybak MJ, et al. Comment on: AUCs and 123s: a critical appraisal of vancomycin therapeutic drug monitoring in paediatrics. J Antimicrob Chemother. 2021; 76(9):2486–2488. PMID:
34021756.

46. Dalton BR, Stewart JJ, Dersch-Mills D, Gin A, Dresser LD, Jorgensen SC. AUCs and 123s: a critical appraisal of vancomycin therapeutic drug monitoring in paediatrics-authors’ response. J Antimicrob Chemother. 2021; 76(9):2488–2489. PMID:
34245275.

47. Jorgensen SC, Spellberg B, Shorr AF, Wright WF. Should therapeutic drug monitoring based on the vancomycin area under the concentration-time curve be standard for serious methicillin-resistant
Staphylococcus aureus infections?-no. Clin Infect Dis. 2021; 72(9):1502–1506. PMID:
33740050.

48. Murphy ME, Tang Girdwood S, Goldman JL, Scheetz MH, Downes KJ. Precision dosing of vancomycin: in defence of AUC-guided therapy in children. J Antimicrob Chemother. 2021; 76(10):2494–2497. PMID:
34096598.

49. Hanai Y, Takahashi Y, Niwa T, Mayumi T, Hamada Y, Kimura T, et al. Clinical practice guidelines for therapeutic drug monitoring of teicoplanin: a consensus review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J Antimicrob Chemother. 2022; 77(4):869–879. PMID:
35022752.

50. Ueda T, Takesue Y, Nakajima K, Ichiki K, Doita A, Wada Y, et al. Enhanced loading regimen of teicoplanin is necessary to achieve therapeutic pharmacokinetics levels for the improvement of clinical outcomes in patients with renal dysfunction. Eur J Clin Microbiol Infect Dis. 2016; 35(9):1501–1509. PMID:
27278654.

51. Ueda T, Takesue Y, Nakajima K, Ichiki K, Ishikawa K, Takai Y, et al. Clinical efficacy and safety in patients treated with teicoplanin with a target trough concentration of 20 μg/mL using a regimen of 12 mg/kg for five doses within the initial 3 days. BMC Pharmacol Toxicol. 2020; 21(1):50. PMID:
32641110.

52. Matthews PC, Chue AL, Wyllie D, Barnett A, Isinkaye T, Jefferies L, et al. Increased teicoplanin doses are associated with improved serum levels but not drug toxicity. J Infect. 2014; 68(1):43–49. PMID:
24012820.

53. Seki M, Yabuno K, Miyawaki K, Miwa Y, Tomono K. Loading regimen required to rapidly achieve therapeutic trough plasma concentration of teicoplanin and evaluation of clinical features. Clin Pharmacol. 2012; 4:71–75. PMID:
23236257.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download