2. Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature. 2021; 600(7887):21. PMID:
34824381.

4. Madhi SA, Kwatra G, Myers JE, Jassat W, Dhar N, Mukendi CK, et al. Population immunity and Covid-19 severity with omicron variant in South Africa. N Engl J Med. 2022; 386(14):1314–1326. PMID:
35196424.

5. Modes ME, Directo MP, Melgar M, Johnson LR, Yang H, Chaudhary P, et al. Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (delta) and B.1.1.529 (omicron) variant predominance - one hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(6):217–223. PMID:
35143466.

6. Kim MK, Lee B, Choi YY, Um J, Lee KS, Sung HK, et al. Clinical characteristics of 40 patients infected with the SARS-CoV-2 omicron variant in Korea. J Korean Med Sci. 2022; 37(3):e31. PMID:
35040299.

7. Chua F, Armstrong-James D, Desai SR, Barnett J, Kouranos V, Kon OM, et al. The role of CT in case ascertainment and management of COVID-19 pneumonia in the UK: insights from high-incidence regions. Lancet Respir Med. 2020; 8(5):438–440. PMID:
32220663.

8. Society of Thoracic Radiology. STR/ASER COVID-19 position statement. Updated 2020. Accessed March 12, 2020.
https://thoracicrad.org/
.
9. Machnicki S, Patel D, Singh A, Talwar A, Mina B, Oks M, et al. The usefulness of chest CT imaging in patients with suspected or diagnosed COVID-19: a review of literature. Chest. 2021; 160(2):652–670. PMID:
33861993.

10. Kanne JP, Bai H, Bernheim A, Chung M, Haramati LB, Kallmes DF, et al. COVID-19 imaging: what we know now and what remains unknown. Radiology. 2021; 299(3):E262–E279. PMID:
33560192.
11. Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020; 295(3):200463. PMID:
32077789.

12. Vijayakumar B, Tonkin J, Devaraj A, et al. CT lung abnormalities after COVID-19 at 3 months and 1 year after hospital discharge. Radiology. 2022; 303(2):444–454. PMID:
34609195.
13. Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020; 296(2):E55–E64. PMID:
32191587.
14. Pan F, Yang L, Liang B, Ye T, Li L, Li L, et al. Chest CT patterns from diagnosis to 1 year of follow-up in patients with COVID-19. Radiology. 2022; 302(3):709–719. PMID:
34609153.

15. Wu Q, Zhong L, Li H, Guo J, Li Y, Hou X, et al. A follow-up study of lung function and chest computed tomography at 6 months after discharge in patients with coronavirus disease 2019. Can Respir J. 2021; 2021:6692409. PMID:
33628349.
16. Fabbri L, Moss S, Khan FA, et al. Parenchymal lung abnormalities following hospitalisation for COVID-19 and viral pneumonitis: a systematic review and meta-analysis. Thorax. 2023; 78(2):191–201. PMID:
35338102.
17. Liang L, Yang B, Jiang N, Fu W, He X, Zhou Y, et al. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci. 2020; 35(47):e418. PMID:
33289374.
20. Kikkenborg Berg S, Palm P, Nygaard U, Bundgaard H, Petersen MN, Rosenkilde S, et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0-14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. 2022; 6(9):614–623. PMID:
35752194.
21. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008; 246(3):697–722. PMID:
18195376.
22. Jeong YJ, Nam BD, Yoo JY, Kim KI, Kang H, Hwang JH, et al. Prognostic implications of CT feature analysis in patients with COVID-19: a nationwide cohort study. J Korean Med Sci. 2021; 36(8):e51. PMID:
33650333.
23. Yoon SH, Lee JH, Kim BN, Chest CT. Findings in hospitalized patients with SARS-CoV-2: delta versus omicron variants. Radiology. 2023; 306(1):252–260. PMID:
35762887.
24. Tsakok MT, Watson RA, Saujani SJ, Kong M, Xie C, Peschl H, et al. Reduction in chest CT severity and improved hospital outcomes in SARS-CoV-2 omicron compared with delta variant infection. Radiology. 2023; 306(1):261–269. PMID:
35727150.
25. Sun D, Li X, Guo D, Wu L, Chen T, Fang Z, et al. CT quantitative analysis and its relationship with clinical features for assessing the severity of patients with COVID-19. Korean J Radiol. 2020; 21(7):859–868. PMID:
32524786.
26. Kang M, Hong KS, Chikontwe P, Luna M, Jang JG, Park J, et al. Quantitative assessment of chest CT patterns in COVID-19 and bacterial pneumonia patients: a deep learning perspective. J Korean Med Sci. 2021; 36(5):e46. PMID:
33527788.
27. Delshad M, Tavakolinia N, Pourbagheri-Sigaroodi A, Safaroghli-Azar A, Bagheri N, Bashash D. The contributory role of lymphocyte subsets, pathophysiology of lymphopenia and its implication as prognostic and therapeutic opportunity in COVID-19. Int Immunopharmacol. 2021; 95:107586. PMID:
33765611.
28. Chen LD, Zhang ZY, Wei XJ, Cai YQ, Yao WZ, Wang MH, et al. Association between cytokine profiles and lung injury in COVID-19 pneumonia. Respir Res. 2020; 21(1):201. PMID:
32727465.

29. Zhang J, Meng G, Li W, Shi B, Dong H, Su Z, et al. Relationship of chest CT score with clinical characteristics of 108 patients hospitalized with COVID-19 in Wuhan, China. Respir Res. 2020; 21(1):180. PMID:
32664991.
30. Pan P, Du X, Zhou Q, Cui Y, Deng X, Liu C, et al. Characteristics of lymphocyte subsets and cytokine profiles of patients with COVID-19. Virol J. 2022; 19(1):57. PMID:
35346253.
31. Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020; 11:1708. PMID:
32754163.
32. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022; 386(16):1532–1546. PMID:
35249272.
33. Tian D, Song Y, Zhang M, Pan Y, Ge Z, Zhang Y, et al. Genomic, immunological, and clinical analysis of COVID-19 vaccine breakthrough infections in Beijing, China. J Med Virol. 2022; 94(5):2237–2249. PMID:
35112366.
34. Li Y, Wang X, Shen XR, Geng R, Xie N, Han JF, et al. A 1-year longitudinal study on COVID-19 convalescents reveals persistence of anti-SARS-CoV-2 humoral and cellular immunity. Emerg Microbes Infect. 2022; 11(1):902–913. PMID:
35240947.

35. Ye L, Hu B, Lin S, Chen M, Fang Y, He S. Dynamic changes in lung function and imaging in patients with COVID-19. Can J Infect Dis Med Microbiol. 2022; 2022:1728446. PMID:
35280351.
36. Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021; 9(7):747–754. PMID:
33964245.
37. Zheng Y, Wang L, Ben S. Meta-analysis of chest CT features of patients with COVID-19 pneumonia. J Med Virol. 2021; 93(1):241–249. PMID:
32579236.
38. Zhong L, Zhang S, Wang J, Zhao X, Wang K, Ding W, et al. Analysis of chest CT results of coronavirus disease 2019 (COVID-19) patients at first follow-up. Can Respir J. 2020; 2020:5328267. PMID:
33204376.
39. Pogatchnik BP, Swenson KE, Sharifi H, Bedi H, Berry GJ, Guo HH. Radiology-pathology correlation demonstrating organizing pneumonia in a patient who recovered from COVID-19. Am J Respir Crit Care Med. 2020; 202(4):598–599. PMID:
32609531.

40. Francone M, Iafrate F, Masci GM, Coco S, Cilia F, Manganaro L, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020; 30(12):6808–6817. PMID:
32623505.
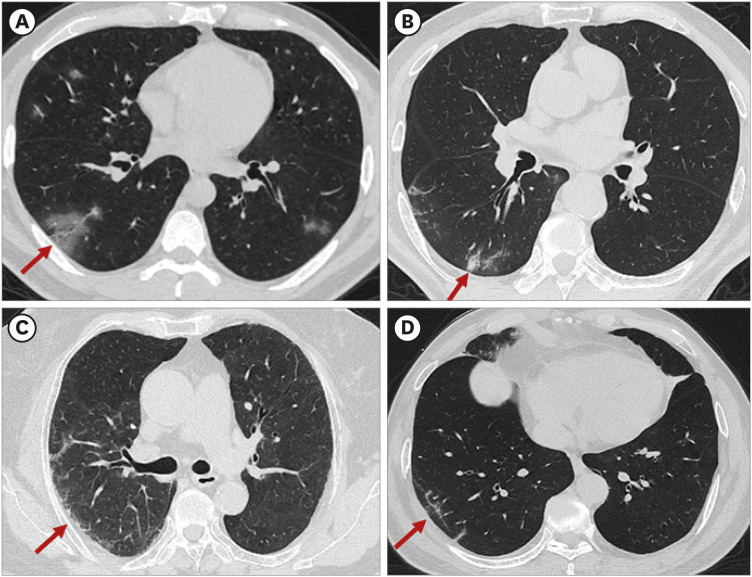
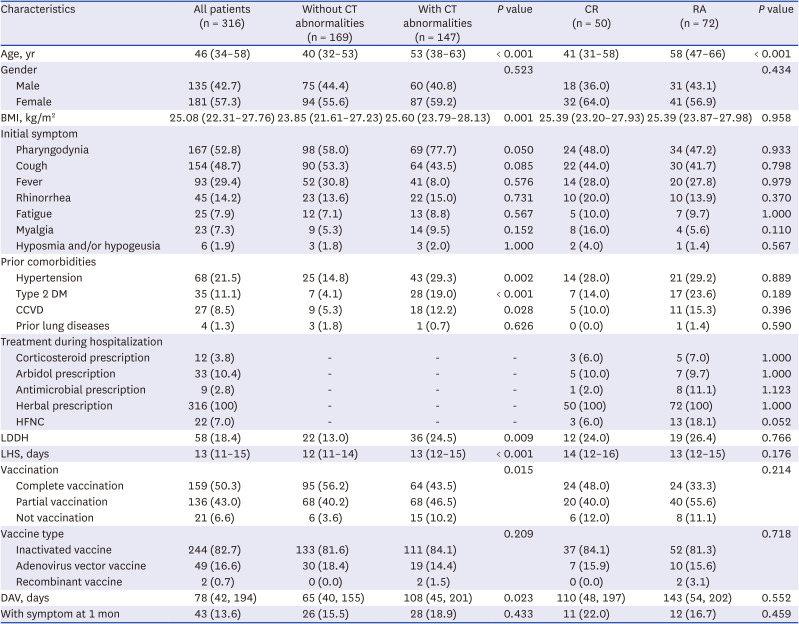
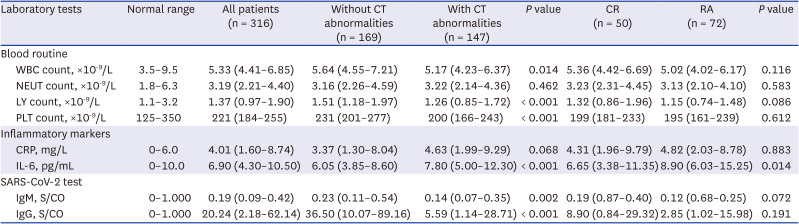
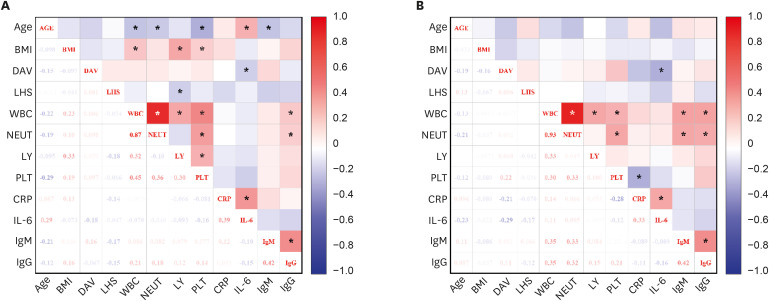




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download