INTRODUCTION
Exposure of young children to general anesthesia is a considerable concern for parents, physicians, and researchers. Since 2017, the U.S. Food and Drug Administration has warned of potential neuronal damage from repeated and lengthy exposure to general anesthesia in children younger than 3 years.
1 In the United States, 14.9% of children under the age of 3 were exposed to general anesthesia more than once a year, and in the United Kingdom, 5% of children under the age of 6 received general anesthesia every year.
23 In addition, according to Statistics Korea, about 0.5% of children younger than 5 years-old received one of 26 major types of surgery during 2019 (836.19 per 100,000 children aged 0 to 1 years-old; 456.24 per 100,000 for children aged 1 to 4 years-old), including heart surgery, hernia surgery, and tonsillectomy.
4 The use of anesthetics is typically necessary in these important medical procedures.
In several preclinical studies, general anesthesia induced neuronal damage and developmental neurotoxicity in the developing brain from the third stage of gestation to approximately 3 years old in humans.
56 Changes in brain tissue structure have been explained by the maturational-stage vulnerability that suppression of neurotrophic synaptic signaling leads to apoptosis.
7 The impairment of the hippocampus and frontal cortex could induce memory and behavioral problems, including attention deficit hyperactivity disorder (ADHD).
8 However, clinical studies have shown conflicting results between early exposure to general anesthesia and neurodevelopmental or behavioral outcomes, depending on the number of exposures, study design, and measuring tools for neurodevelopmental status.
91011 In South Korea, a developmental screening test has been conducted annually for all children aged 1 to 6 years from 2007 to the present. Furthermore, since health insurance has been implemented for the entire nation, we aimed to assess the association between exposure to general anesthesia from infant to childhood and ADHD using national health administrative data and neurodevelopmental screening tests of approximately 900,000 children born in 2008 and 2009 in South Korea.
METHODS
Study design and setting
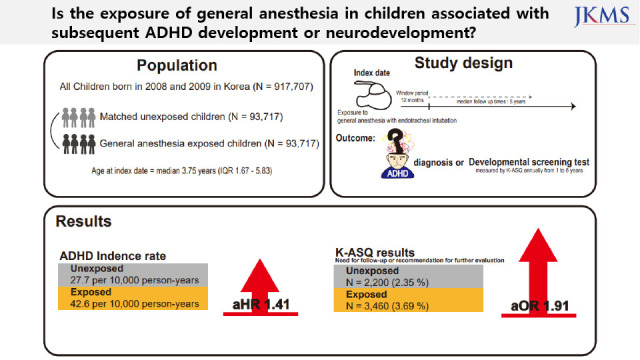
This nationwide administrative cohort included the data of 917,707 children who were born between January 1, 2008 and December 31, 2009.
12 All participants were followed until they were ineligible for health care services or until December 31, 2017. The National Health Insurance Service (NHIS) provides approximately 97% of the residents with healthcare access. The National Health Screening Program for Infants and Children (NHSPIC) is a population surveillance program of 7 rounds for all health insurance subscribers from 4 to 72 months old. The index date for each participant was defined as the date of surgery upon receipt of general anesthesia with endotracheal intubation (ETI). The window period was set at 12 months after the index date.
Data sources
All data were obtained from the NHIS and NHSPIC. The NHIS includes information on healthcare utilization, residential area at birth, and economic status. The NHSPIC included a general health questionnaire answered by parents and physical examination in all rounds and a developmental screening test of the Korean-Ages and Stages Questionnaire (K-ASQ) in the second to seventh rounds (annually from the age of 1 to 6 years). The status of prematurity and birth weight were acquired from the parent-answered questionnaire of the NHSPIC. The 10th revision of the International Classification of Diseases (ICD-10 code) of perinatal conditions, diagnosed disease before the index date, and prescribed drug classification codes were obtained from the NHIS.
Study population
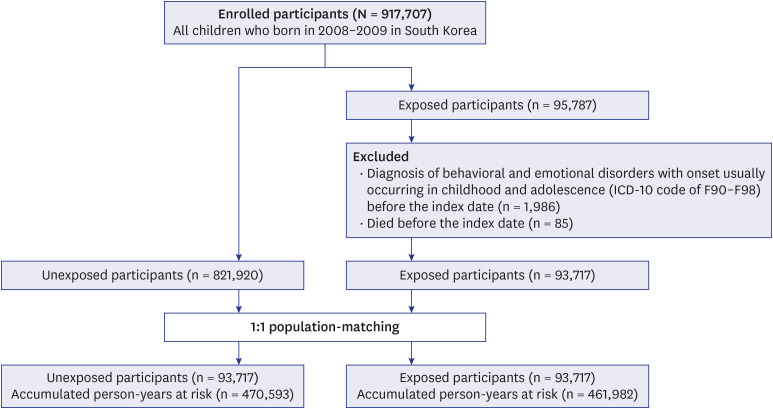
Among the 917,707 children, those who died and those who were diagnosed with behavioral and emotional disorders before their index date were excluded. The 915,637 eligible children were divided into those who received surgery under general anesthesia with ETI (exposed group, n = 93,717) and those who never received surgery (unexposed group, n = 821,920) (
Fig. 1). For analysis of the main cohort, we conducted a comparison using a 1:1 matched unexposed cohort with density sampling. Overall, there were 93,717 children in each group. For analysis of a secondary cohort, 1:1 propensity score (PS) matching was performed using 30,341 children in each group (
Supplementary Fig. 1).
Fig. 1
Study design.
ICD-10 = 10th revision of the International Classification of Diseases.

Exposure
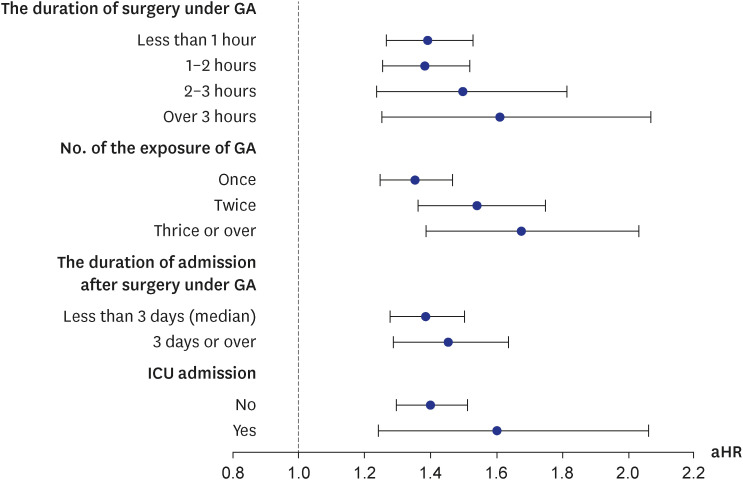
The exposure of interest was the first event of general anesthesia with ETI during surgery, which was recorded from treatment codes in the NHIS. To estimate the effect of the intensity of general anesthesia with ETI and a participant’s condition after induction, exposure was stratified according to: 1) duration of general anesthesia (less than 1 hour, 1–2 hours, 2–3 hours, or more than 3 hours); 2) number of general anesthesia procedures (1, 2, 3 or more); 3) duration of admission after receipt of general anesthesia (less than 3 days [median] or 3 days or more); and 4) admission to an intensive care unit (ICU) after receipt of general anesthesia (yes or no).
Outcomes
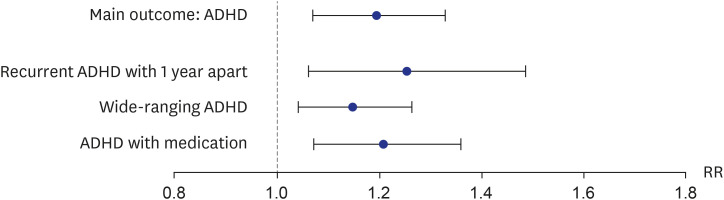
All outcomes were prespecified. The primary outcome of this study was ADHD. Based on previous validation studies, ADHD was defined as at least a principal diagnosis of ICD-10 code F90.X after 72 months old. Using a principal diagnosis based on the ICD-10 code to define ADHD had high sensitivity (0.96 to 0.97), specificity (0.98 to 0.99), and positive predictive value (0.83 to 0.98).
13
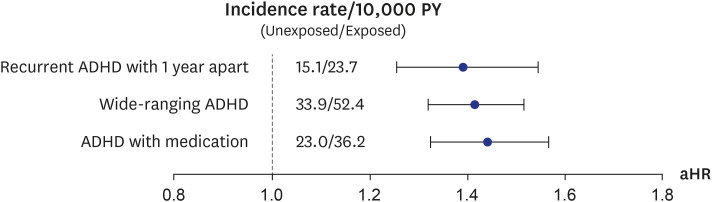
For the sensitivity analysis, alternative definitions of ADHD were defined. First, a recurrent ADHD 1 year apart was defined as 2 principal diagnoses of ICD-10 codes F90.X after 72 months old that were 1 year apart. Second, a wide-ranging ADHD was defined as at least a principal or second diagnosis of ICD-10 code F90.X after 72 months old. Third, ADHD with medication was defined as at least a principal diagnosis of ICD-10 code F90.X after 72 months old with a prescription of methylphenidate.
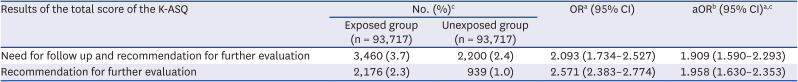
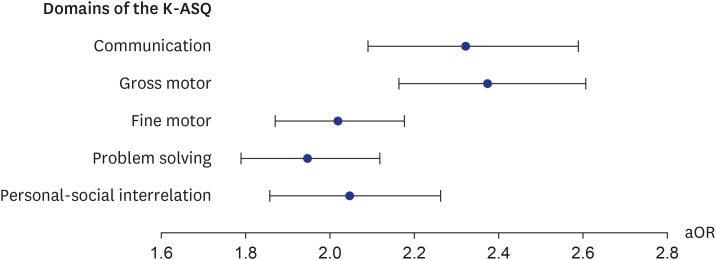
In addition, the secondary outcome was the association with the K-ASQ results to estimate neurodevelopment after exposure to general anesthesia with ETI. The K-ASQ consists of 5 domains: communication, gross motor skills, fine motor skills, problem-solving, and personal-social interrelation. The results of each domain of the K-ASQ were stratified as appropriate, need for follow-up, and recommendations for further evaluation. The results of need for follow-up and recommendation for further evaluation mean scores below −1 standard deviation (SD) for age and below −2 SD for age, respectively. For analysis of the K-ASQ results, the two outcomes of interest were: 1) recommendations for further evaluation and 2) the sum of the need for follow-up and recommendation for further evaluation.
All outcomes were identified for at least 1 year (window period) after the index date.
Covariates
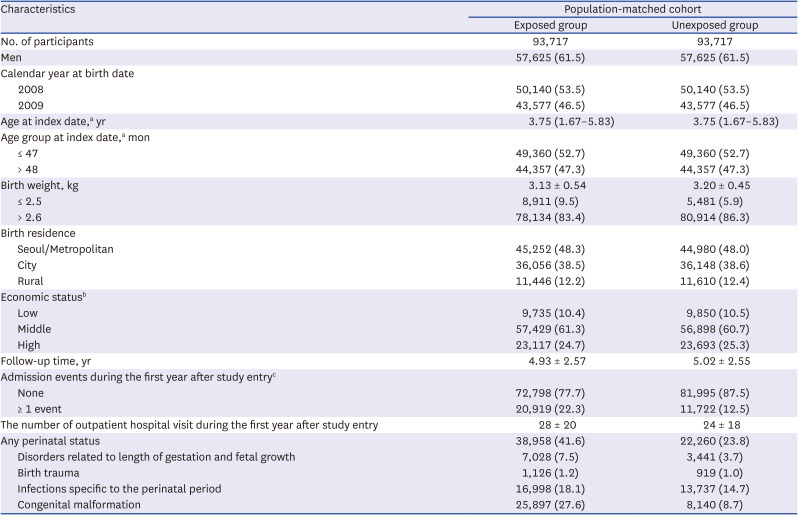
Sociodemographic variables included age, sex, birth weight, residential area at birth, and economic status. Residential areas at birth were divided into Seoul, metropolitan (Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan), urban, and rural areas. Economic status was estimated by using health insurance premiums. Information about any perinatal diseases (including disorders related to length of gestation and fetal growth [ICD-10 code P05.X to P08.X], birth trauma [P10.X to P15.X], infections specific to the perinatal period [P35.X to P39.X], and congenital malformations, deformations, and chromosomal abnormalities [Q00.X to Q99.X]) were assessed.
Statistical analysis
For population-based matching in a main cohort, a subject unexposed to general anesthesia and with ADHD was randomly selected for each exposed subject, and the year of birth and sex of both subjects were individually matched. Matched individuals in the unexposed group were followed-up to investigate whether they were subsequently exposed to general anesthesia. If they were exposed to general anesthesia, they were transferred to the exposed group. In addition, a second cohort was established using 1:1 PS matching to balance the baseline characteristics of the exposed and unexposed groups. The PS was estimated using multivariable logistic regression with baseline variables of sex, year of birth, birth residence, economic status, perinatal diseases, and birth weight. The 1:1 PS matching was performed using the Mahalanobis algorithm with a caliper of 0.01. Differences between the two groups were evaluated using standardized differences, and a standardized difference greater than 10% was considered significant.
We excluded the first year of follow-up from the analysis to minimize the possibility of inverse causality or surveillance bias, because participants who receive anesthesia typically utilize more health care resources during the first month after this procedure.
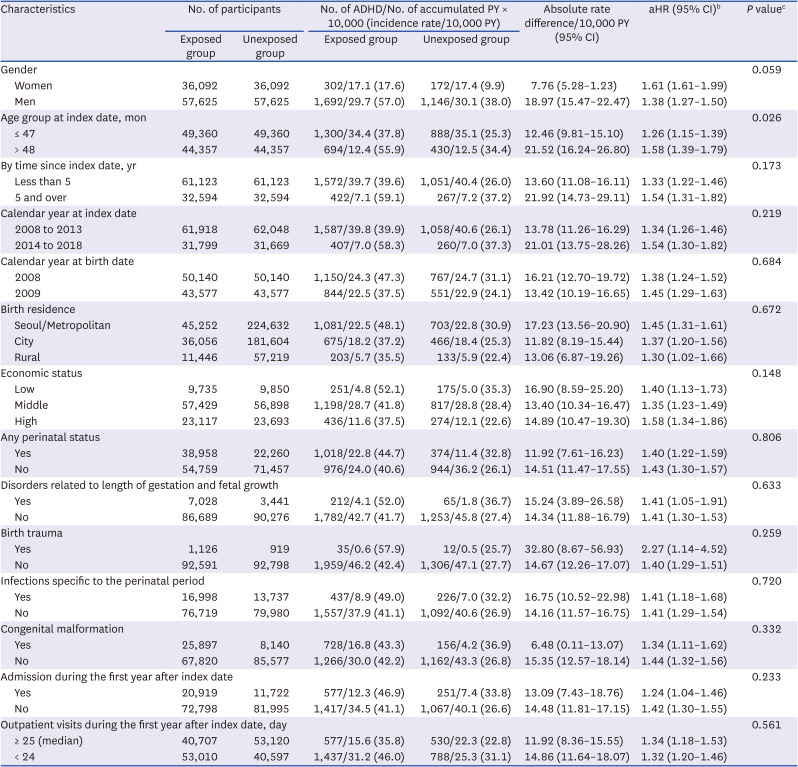
A Cox proportional hazards model was used to assess the association between general anesthesia with ETI and development of ADHD in the main cohort. Hazard ratios (HRs) and 95% confidence intervals (CIs) of ADHD in relation to previous exposure, using time after the index date as the underlying time scale, were calculated. All participants were traced from the index date, and censored on the first diagnosis of ADHD, death, or the end of the study (December 31, 2017), whichever came first. The absolute rate differences with 95% CIs were also calculated. All analyses were adjusted for age at the index date; calendar year of birth (2008 or 2009); birth weight; birth residence (Seoul, metropolitan, city, or rural area); economic status (low, middle, or high); and perinatal diseases. In addition, the HRs were separately calculated for sex, age group at index date (48 months), time since index date (5 years), calendar year at index date, calendar year at birth date, birth residence, income quintile, perinatal status (ICD-10 P or Q code), history of admission during the first year after study entry, and frequency of outpatient visits within a year after the index date. We assessed differences in HRs by introducing an interaction term to the Cox model. In the second cohort, modified Poisson regression were used to estimate crude risk ratios (RRs) and their 95% CIs of ADHD in the exposed group compared to the unexposed group.
To assess the association between general anesthesia with ETI and results of the K-ASQ in children, odds ratios (ORs) and their 95% CIs were calculated by using generalized estimated equations with the logit link function. For intra-subgroup OR comparisons, the log OR difference between the strata was used to calculate the z-score and P values. Two-tailed P values of less than 0.01 were interpreted as statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Ethics statement
This study was reviewed and approved by the Institutional Review Board of the Korea National Institute for Bioethics Policy (P01-201603-21-005). No informed consent was required from patients due to the nature of the public data from NHIS of the use of de-identified individual data for research purposes.
DISCUSSION
Our results showed that general anesthesia with ETI during childhood was associated with an increased risk of ADHD and was potentially associated with adverse neurodevelopment in preschoolers based on screening test results. In addition, we found no difference in the effects of general anesthesia with ETI on ADHD in subjects who had different demographic characteristics, in that the associations were statistically significant in all demographic groups. We also assessed the association of ADHD with the duration of general anesthesia with ETI, number of general anesthesia with ETI procedures, duration of admission after general anesthesia with ETI, and ICU admission after general anesthesia with ETI. These results indicated that a longer exposure to general anesthesia with ETI, more procedures of general anesthesia with ETI, longer hospital stay, and the need for ICU treatment after general anesthesia with ETI led to stronger associations with ADHD. Furthermore, based on the K-ASQ screening test, general anesthesia with ETI was also associated with a need for follow-up or recommendation for further evaluation.
Since the 2010s, the association between general anesthesia exposure and ADHD has been reported in clinical studies; however, the reported associations have been inconsistent. In a U.S. cohort of 185,002 children, anesthesia exposure was associated with an increased incidence of ADHD in children under 5 years old, with more exposures associated with greater risk.
14 In addition, a study in Taiwan reported no increased risk of ADHD in children under 3 years-old following general anesthesia (aHR [95% CI], 1.10 [0.80–1.51]) after adjusting for gestational age, sex, living area, parents’ economic status, parents’ occupations, and comorbid health conditions. However, male sex, living in an urban area, congenital abnormalities, multiple exposures to general anesthesia, and receipt of general anesthesia for more than 3 hours were significantly related to ADHD.
15 Other research from Taiwan showed that general anesthesia was not associated with later development of ADHD in children under 3 years even after multiple exposures.
16 Even when using the same database, different results were shown based on the study setting or definition of outcomes. While the reported effects of a single exposure to general anesthesia in young children on ADHD development were inconclusive, most clinical studies have shown consistent results that multiple exposures to general anesthesia was associated with ADHD in young children.
In a meta-analysis of the association between general anesthesia and neurodevelopment in children, the risk of neurodevelopmental impairment was increased following general anesthesia exposure in children under 5 years old (pooled HR [95% CI], 1.25 [1.13–1.38]). However, a recent systematic review reported that there is no conclusive evidence that a single and short anesthesia exposure in infancy had an effect on neurodevelopment.
17 An international randomized controlled trial, which is the most robust study to date, assessed the associations between adverse neurodevelopment at 2 and 5 years of age and general anesthesia exposure and compared them to those after awake-regional anesthesia exposure for inguinal hernia repair. In that study, neurodevelopment was assessed by the Bayley Scales of Infant Development-III at 2 years old and the Wechsler Preschool and Primary Scale of Intelligence-III Full Scale Intelligence Quotient score at 5 years old. Their results showed no significant association between a single general anesthesia procedure lasting less than 1 hour for herniorrhaphy and neurodevelopment compared to awake-regional anesthesia at the age of 2 years-old and 5 years-old.
918 In contrast, our study showed that receipt of general anesthesia with ETI was potentially associated with adverse results in a developmental screening test at the preschool age. Further studies will be needed to confirm the effect of general anesthesia on neurodevelopment in children.
Brain structure and function develop and mature throughout childhood. Specifically, the first 2 years are critical for sensory development, the period before the age of 5 years is important for language and motor development, and later periods are important for development of higher cognition.
19 A previous animal study found that administration of general anesthesia during a period analogous to infancy and young childhood altered the prefrontal cortex and reduced axonal connections,
20 and that these effects persisted into the period analogous to adulthood.
15 The prefrontal cortex plays an important role in the pathophysiology of ADHD, and the effects of general anesthesia on the prefrontal cortex are apparently dose-dependent and persist into adulthood. Therefore, although the results of this animal study cannot be verified in humans, they provide a possible explanation for the relationship between receipt of general anesthesia during childhood and ADHD. In addition, exposure to anesthetic agents might induce developmental neurotoxicity and accelerate neurodegeneration in young children. In vitro and in vivo studies have reported that exposure to anesthetic agents have dose-dependent effects on various neuronal transmission systems and increase neuronal cell death.
21 Furthermore, excitotoxicity is regarded as one of the mechanisms of neuronal cell death caused by anesthetic agents. The Na
+-K
+-2Cl
− cotransporter isoform 1 (NKCC1) generates a chloride influx that leads to depolarization of γ-aminobutyric acid (GABA
A) receptor-mediated responses in immature neurons, and is immature until the first year after birth. Although GABA is an inhibitory agent in the mature brain, it is an excitatory agent during the developmental period when the chloride transporter is immature.
2223 Therefore, anesthetic agents could stimulate rather than inhibit immature neurons in young children, and this could lead to excitotoxicity. Lastly, exposure to anesthetic agents has an effect on epigenetic modulation of transcription, which could globally affect neurodevelopment and synaptogenesis.
24
The risk for the neuropsychiatric disorders after general anesthesia is similar for different age groups from birth to the age of 5 years-old.
25 Most studies that examined the effects of general anesthesia in children under the age of 3 to 5 years reported neurodevelopmental alterations or neuropsychiatric disorders
26 because this period is a critical time for neurodevelopment. However, the period after the age of 5 years is also important for higher cognition, sensitivity, and neurologic plasticity.
19 The present study showed that receipt of general anesthesia with ETI before or after the age of 4 years was associated with a subsequent diagnosis of ADHD. Future studies will be needed to confirm this association.
The present study is a nationwide general pediatric population study in which data had representative characteristics and the results can be generalized. Furthermore, the K-ASQ is a validated developmental screening test for Korean children; therefore, this database was appropriate for assessing the association between general anesthesia exposure and adverse neurodevelopment in children. In addition, we analyzed the associations between general anesthesia and ADHD according to demographic and clinical characteristics and the concentration of general anesthesia.
However, our study had some limitations. First, this study had no information regarding the type and dosage of anesthetic agents that might affect neurodevelopmental risk. To evaluate the risk depending on the concentration of anesthesia, we analyzed data from insurance receipts for procedures, including intubation for general anesthesia that determined the duration of exposure. In addition, we relied on the definitions of ADHD according to ICD-10 and drug classification codes from the administrative data. Thus, we attempted to confirm the results using various definitions of ADHD. Second, the developmental outcomes from the K-ASQ are screening data that recommend further developmental tests. This score cannot show the diagnostic information of neurodevelopment, such as the intelligence quotient or developmental age. Third, we could not assess potential unmeasured confounders that may have affected the development of ADHD or neurodevelopment, such as parental education level, genetic background, or family history. Finally, because this was an observational study, we could not infer causal relationships.
In conclusion, exposure to general anesthesia with ETI in children is associated with an increased risk of ADHD and adverse results of neurodevelopmental screening test. We must recognize the possible neurodevelopmental risk resulting from general anesthesia exposure, inform patients and parents regarding this risk, and emphasize the importance of close monitoring of mental health. However, the risk from anesthesia exposure is not superior to the importance of medical procedures. Specific research is needed for the development of safer anesthetic drugs and doses.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download