INTRODUCTION
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is the most common form of autoimmune encephalitis.
1 Common initial symptoms include fever, memory loss, and psychiatric symptoms, which are easily misdiagnosed as primary psychiatric manifestations. Within a few days, neurological manifestations begin to appear, such as altered consciousness, seizures, dyskinesia, and autonomic dysregulation.
234
Anti-NMDAR encephalitis has been reported predominantly in young women and is commonly associated with underlying ovarian teratomas. In an observational cohort study, 94% of tumors related to anti-NMDAR encephalitis were ovarian teratomas, and only 2% were caused by extra-ovarian teratomas.
56 For anti-NMDAR encephalitis treatment, immunotherapy, and removal of immunologic triggers, such as teratomas or other tumors, are known to be effective, if applicable.
57 Here, we report a case of a patient with NMDAR encephalitis who presented with teratomas in the mediastinum and bilateral ovaries simultaneously.
CASE DESCRIPTION
In July 2019, a 28-year-old woman with no relevant medical history was admitted to the neurology department because of headache, agitation, and fluctuations in responsiveness for the last seven days. The patient’s initial vital signs were stable. Neurologic examination on admission revealed no positive signs, and the patient showed intact consciousness and responsiveness.
After admission, the patient started to shout and showed lip-smacking during mealtime. She was conscious but unresponsive for periods lasting several minutes. She repeated sentences, such as “listen to me, please” or “I do not know.” After an injection of lorazepam (2 mg), the patient recovered and was able to speak, but she did not remember the described event. On the third day at the hospital, the patient presented generalized tonic-clonic seizures but did not recover to baseline consciousness and was transferred to an intensive care unit.
Laboratory test results showed a total white blood cell (WBC) count of 8.71 × 103/L. C-reactive protein levels, blood coagulation test results, and erythrocyte sedimentation rate were normal. Lumbar puncture revealed a cerebrospinal fluid (CSF) pressure of 150 mmH2O; routine CSF analysis, a WBC count of 1/µL, and the biochemical test, normal CSF biochemistry. Electroencephalography (EEG) showed slow waves without epileptiform discharges or ictal findings. Neither enhanced brain magnetic resonance imaging nor electrocardiography revealed any abnormalities.
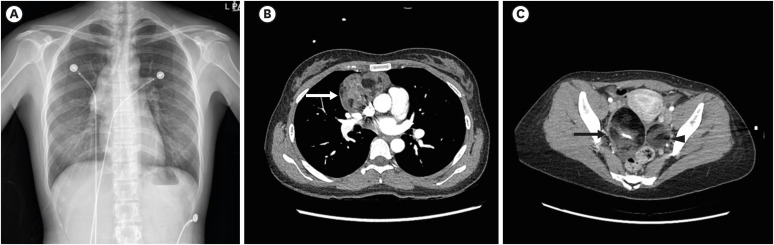
Chest radiography revealed a large anterior mediastinal mass (
Fig. 1A), and a chest computed tomography (CT) scan showed a 7.3 cm anterior mediastinal cystic tumor (
Fig. 1B). Tumor marker levels (human chorionic gonadotropin, alpha-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 19-9, cancer antigen 125, and neuron-specific enolase) were normal. For the potential identification of autoimmune encephalitis, tests were performed on the serum and CSF for autoimmune antibodies. Anti-NMDAR antibody was detected in both the serum and CSF. The chest CT confirmed the mediastinal mass, and abdominopelvic CT was performed to confirm the presence of additional lesions, despite the risk of additional radiation exposure; this revealed mature cystic teratomas in both ovaries (
Fig. 1C). In addition, F
18-fluorodeoxyglucose torso positron emission tomography-CT showed anterior mediastinum and bilateral adnexa masses which were suspected of being teratomas.
Fig. 1
Radiologic findings of mediastinal and ovarian masses. (A) Chest X-ray shows an anterior mediastinal mass. (B) Chest computed tomographic scan reveals a large mixed-density mass (9 × 7.5 × 4.7 cm3) (arrow). (C) Ovarian teratomas: abdominopelvic computed tomographic scan reveals mixed-density masses suggestive of mature cystic teratomas. Right (arrow): 6.5 × 4.7 × 1.5 cm3; Left (arrowhead): 4 × 3.7 × 0.7 cm3. The figures are published under agreement of the patient.
Regarding treatment, surgical management was planned to remove the masses on the mediastinum and both ovaries. Excision of the mediastinal mass and ovarian cystectomy were performed simultaneously. The size of the mediastinal mass, right ovarian cyst, and left ovarian cyst were 9 × 7.5 × 4.7 cm, 7.5 × 5.5 cm, and 4.3 × 3.6 cm, respectively.
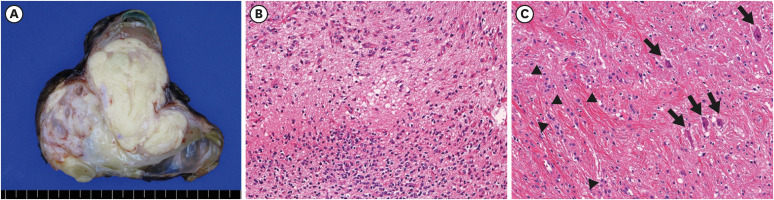
The mediastinal mass was a lobulated and solid mass with abundant yellow fat tissue and several cystic spaces (
Fig. 2A). Microscopic examination revealed a mature teratoma composed predominantly of fat tissue with other various minor components, such as cartilage, skeletal muscle, keratinized squamous epithelium, skin appendage, glial and salivary gland tissue, bronchial and intestinal mucosa. Glial tissue comprised less than 1% of the whole mass, and this was composed of oligodendroglial cells, reactive astrocytes, and neurophils (
Fig. 2B). The right and left ovarian cysts were unilocular cysts containing hair and sebum. Both ovarian cysts were mature cystic teratomas comprising keratin, squamous epithelium, adipocyte and sweat and sebaceous glands. The neuronal component was minimally present in the right ovarian cyst, composed of a few neurons and some astrocytes (
Fig. 2C).
Fig. 2
Pathological features of the mediastinal mass and right ovarian cyst. (A) The anterior mediastinal mass was a large, solid mass with abundant yellow fat tissue and several cysts. (B) Glial element of anterior mediastinal mass was focally present and contained oligodendroglial cells (lower one-third of the figure) and reactive astrocytes (upper portion) in the background of neutrophils (×200). (C) Neuronal element of right ovarian cyst was minimally identified, including a few neurons (arrows) and some astrocytes (arrowheads, ×200).
Immunotherapy was also administered. As first-line treatment, intravenous methylprednisolone (1 g/day) was administered for 5 days and intravenous immunoglobulin 2.5 g/day was administered for 7 days. The patient gradually recovered consciousness and was extubated on hospital day 19. However, on hospital day 20, the patient showed apnea and decreased oxygen saturation; therefore, intubation and mechanical ventilation were applied, and intravenous methylprednisolone was additionally administered for 5 days. Subsequently, prednisolone was administered orally and tapered slowly until discharge.
The patient also presented with generalized tonic-clonic and focal seizures. The levetiracetam dose was escalated to 1,000 mg twice daily, although intermittent seizure event was still present. Valproic acid (450 mg twice daily) and oxcarbazepine (450 mg twice daily) were added to the regimen. However, because of repetitive seizures, a continuous midazolam infusion was attempted for 4 days. After tapering the continuous midazolam infusion, topiramate (25 mg twice daily) was added on adjusting the antiepileptic drugs. Clinical seizure and ictal EEGs disappeared as assessed by continuous monitoring.
Her mental status gradually recovered and returned to normal levels. At 45 days after admission, the patient still suffered from ataxia and memory deficits but was sufficiently recovered for discharge. During subsequent treatment at the outpatient clinic, her memory loss and ataxia improved. Furthermore, the patient was able to maintain activities of daily living in one month after discharge and returned to work in 3 months. During 24 months of follow-up, no signs of seizures were observed.
DISCUSSION
Anti-NMDAR encephalitis was initially considered as a paraneoplastic syndrome in young women with ovarian teratomas. However, recent studies reported cases of women, as well as men and children, with this condition and the absence of associated tumors.
35 The largest cohort study of patients with anti-NMDAR encephalitis (n = 577) reported that associated tumors were found in 38% of overall patients and 46% of female patients; among them 94% had ovarian teratomas, 2% had extraovarian teratomas and 4% had other tumors types.
5 A few anti-NMDAR encephalitis cases with extraovarian teratomas have been reported in the anterior mediastinum
6891011 and thyroid,
12 suggesting that rapid teratoma detection is key because surgical removal of the primary tumor along with prompt initiation of immunotherapy can achieve improvement and prevent relapse.
Anti-NMDAR encephalitis-associated teratomas are usually found in the ovary or testis, and only 1–3% arise in the mediastinum, head, neck, and thyroid.
6812 Patients with primary multiple organ teratomas in the ovary with mediastinum
10 and liver and mediastinum have been rarely reported.
13 However, there have been no reports of multiple simultaneous teratomas occurring concomitantly with anti-NMDAR encephalitis. Herein, we report the first case of anti-NMDAR encephalitis-associated multiorgan teratomas involving ovary and mediastinum.
Pelvic scans have already been important workup to exclude ovarian tumors in women with anti-NMDAR encephalitis. In this case of anti-NMDAR encephalitis, close evaluation with thorough examination is important for treatment as well. This case highlights the importance of detecting teratomas using a whole-body evaluation. Although simultaneous multiorgan teratomas in NMDAR encephalitis are unusual, whole-body evaluation is imperative to detect multiorgan teratoma in NMDAR encephalitis patients, considering that missed resection of additional teratoma could cause failure of treatment. As such, it is possible to improve clinical manifestation and prevent relapse through early detection and prompt surgical removal and initiation of immunotherapy.
In conclusion, anti-NMDAR encephalitis is a potentially life-threatening autoimmune disorder and whole-body evaluation is essential for excluding the possibility of anti-NMDAR encephalitis-associated multiorgan teratomas and successful treatment.






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download