This article has been
cited by other articles in ScienceCentral.
Escherichia coli Nissle 1917 (EcN) strain is a Gram-negative strain isolated from a young German soldier who were not infected with
Shigella during the endemic period at World War I. The probiotic drug Mutaflor
® using this EcN is available and used in managing gastrointestinal disorders including diarrhea, uncomplicated diverticular disease, and ulcerative colitis (UC) [
1]. As for UC, the relapse rate during 12 months in patients with UC between EcN treated group and mesalazine treated group were statistically equivalent in a randomized, double blind, double dummy trial [
1]. However, there have been few previous studies to demonstrate the mechanism of its probiotic effects [
2]. Also, the anti-inflammatory effects of EcN in mouse colitis models were controversial [
3]. Schultz et al. [
4]~ reported that there was no effect of administration of EcN for 10 days on an acute 2% dextran sulfate sodium (DSS) induced colitis model. On the other hand, Rodríguez-Nogales et al. [
5]~ demonstrated the effect of EcN administration for 26 days on the acute 3% DSS-induced colitis model. We aimed this study to investigated the anti-inflammatory properties of EcN in the DSS murine colitis model and evaluated the related inflammatory responses associated with T helper 17 (Th17), Th1, and regulator T (Treg) cells.
Male C57BL/6 mice (8-week-old) were administered 2.5% (w/v) DSS (MP Biomedicals, Solon, OH, USA) in their drinking water for 6 days, and water was replaced with DSS-free water before euthanization. Animals were randomly assigned to 2 groups; DSS+Veh (phosphate buffered saline [PBS]-treated vehicle) (n=4) and The DSS+EcN groups (n=4). The DSS+Veh group was administered with 0.1 mL PBS, and the The DSS+EcN group was administered with 1×10
8 bacteria in 0.1 mL PBS by oral gavage on days 0, 2, and 5. All mice were sacrificed 8 days after the onset of the study. Mice were monitored for body weight loss, and the entire colon was isolated from the harvested mouse. The colon length was measured between the ileocecal junction and the rectum. Parts of the distal colon were cut into 2 to 3 pieces for periodic acid-Schiff staining and images were obtained using a model BX41 light microscope (Olympus Optical, Tokyo, Japan). Spleens were harvested for flow cytometry, and cells were analyzed with a FACSVerse flow cytometer (BD Biosciences, San Jose, CA, USA) and were analyzed using FlowJo software (Tree Star, Eugene, OR, USA) [
6]. All animal experiments complied with all applicable Korean laws, and were approved by the Institutional Animal Care and Use Committee of Yonsei University Severance Hospital (Seoul, Korea) (IACUC No. 2019-0241). The experiments complied with the approved IACUC guidelines. All results are expressed as standard error of the mean. GraphPad Software (La Jolla, CA, USA) was used for all analyses. The significance of differences between conditions was assessed using Mann-Whitney test.
P-values <0.05 were considered significant.
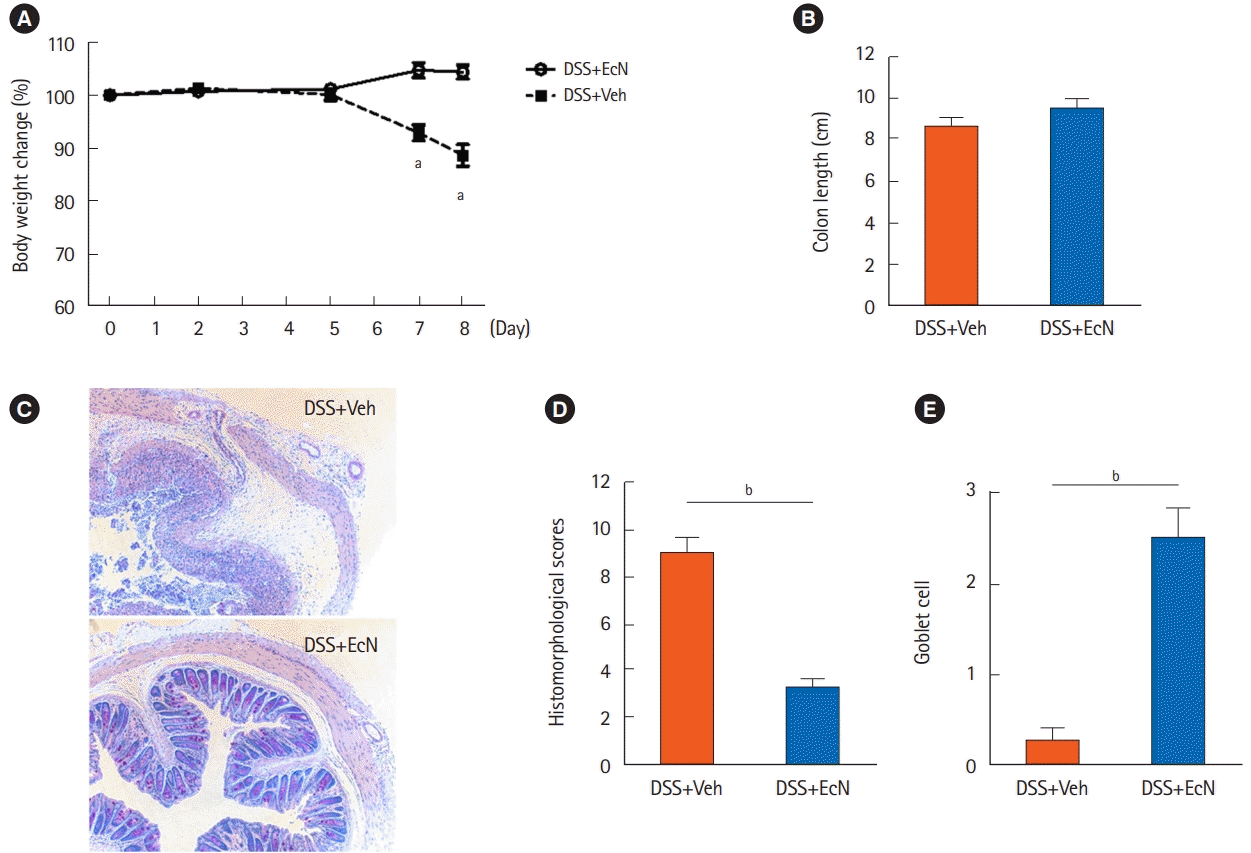
The DSS+EcN group significantly improved body weight compared to the DSS+Veh group (104.4% vs. 88.7%,
P<0.05) (
Fig. 1A). The colon length was numerically longer in the The DSS+EcN group compared to the DSS+Veh group, although this was not statistically significant (9.5 cm vs. 8.6 cm,
P=0.381) (
Fig. 1B). In the histological analysis, histological score was shown to be significantly improved in the The DSS+EcN group compared to DSS+Veh group (3.3 vs. 9.0,
P<0.05) (
Fig. 1C and
D). Also, goblet cell numbers were significantly increased in the The DSS+EcN group compared to DSS+Veh group (2.5 vs. 0.3,
P<0.05) (
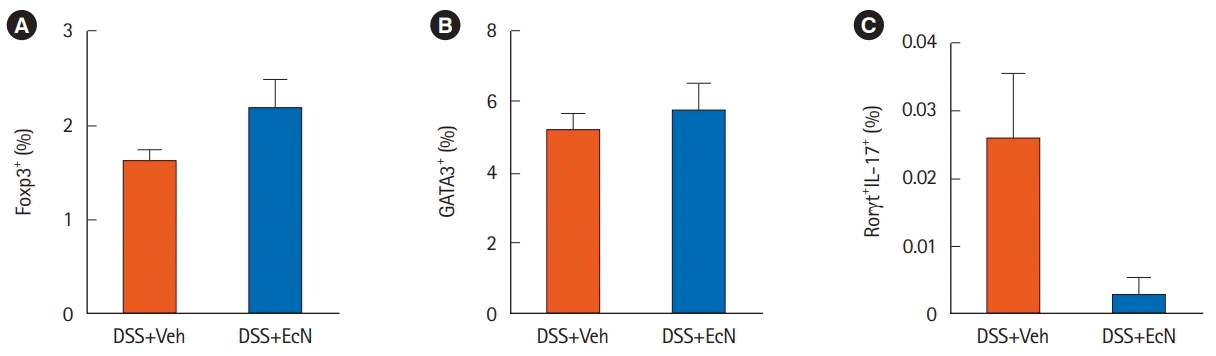
Fig. 1E). We next investigated T cells in splenocytes by flow cytometry. The number of CD4
+Foxp3
+ Treg cell was increased (
P=0.200) and that of CD4
+Rorγt
+IL-17
+ Th17 cells (
P= 0.124) were numerically decreased in the The DSS+EcN group compared to DSS+Veh group, although this was not statistically significant (
Fig. 2).
The several mechanisms of anti-inflammatory properties of EcN were suggested in the previous studies. EcN modulates the gut barrier by upregulating tight junction proteins including zonula occludens (ZO-1 and ZO-2) and antimicrobial factors including microcins [
7]. Furthermore, host-microbe interactions by dendritic cells could modulate innate and adaptive immunity including T lymphocyte responses. The induction of Tregs by probiotics could lead the new therapeutic approach as an alternative to immunosuppressive drugs [
8]. Weise et al. [
9]~ reported that EcN induced the CD4
+Foxp3
+ Treg cells in an allergen- induced mouse dermatitis model. With regard to mouse colitis model, the mechanism modulating γδ T cells by EcN was suggested in the previous study, but the impact of Treg/Th17 responses were not yet evaluated [
9]. We demonstrated the anti-inflammatory properties of EcN in the DSS-induced murine colitis model. Moreover, our results suggest that EcN may modulate the Treg/Th17 responses to maintain gut homeostasis. Further studies investigating the underlying immunologic mechanisms related protective effects of EcN on colitis would be warranted. Further studies on the potential of EcN for protective effects against the development of colorectal cancer would also be valuable [
10]. Finally, more clinical data on its clinical relevance are needed to use this probiotics in the treatment of UC.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download