Abstract
Background/Aims
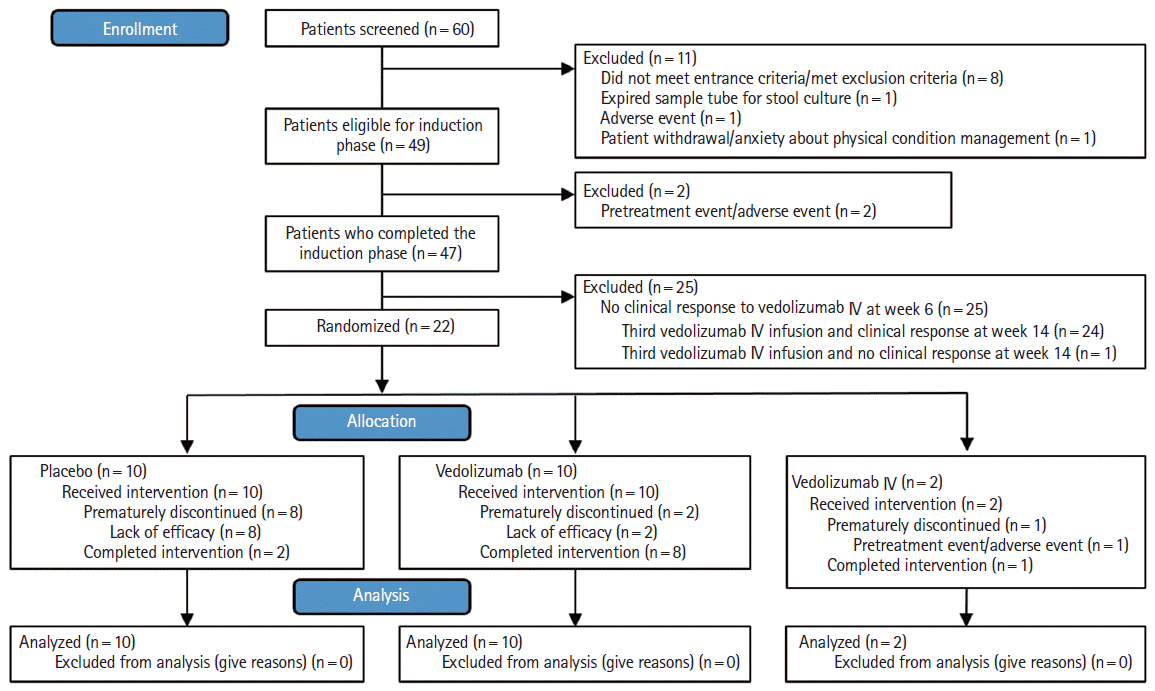
Methods
Results
Conclusions
Notes
Conflict of Interest
Hibi T has received payment for lectures including service on speakers bureaus from Aspen Japan, AbbVie, Ferring, Gilead Sciences, Janssen, JIMRO, Kissei Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku, Pfizer, Takeda Pharmaceutical, and Zeria Pharmaceutical; and has received grants from AbbVie, EA Pharma, JIMRO, Otsuka Holdings, and Zeria Pharmaceutical. Kobayashi T has received personal fees as an advisor from Alfresa Pharma, Covidien, Eli Lilly, Ferring Pharmaceuticals, Janssen, Kyorin Pharmaceutical, Mochida Pharmaceutical, Nippon Kyaku, Pfizer, Takeda Pharmaceutical, Thermo Scientific, AbbVie, Gilead Sciences, Celltrion, and EA Pharma; personal fees for lectures from AbbVie, Pfizer, Astellas, Alfresa Pharma, Cellitron, EA Pharma, Kyorin Pharmaceutical, Nippon Kyaku, Mochida Pharmaceutical, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Zeria Pharmaceutical, Gilead Sciences, Janssen, JIMRO, and Covidien; and grants from JIMRO, AbbVie, EA Pharma, Zeria Pharmaceutical, Kyorin Pharmaceutical, Mochida Pharmaceutical, Thermo Fisher Scientific, Alfresa Pharma, and Nippon Kayaku. Ito H has received payment for lectures including service on speakers bureaus from Mitsubishi Tanabe Pharma, AbbVie, Takeda Pharmaceutical, EA Pharma, Zeria Pharmaceutical, Janssen, Mochida Pharmaceutical, Nippon Kayaku, and Pfizer. Ashida T has received payment for lectures including service on speakers bureaus from Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, and Kissei Pharmaceutical. Yokoyama T has received grants from Mochida Pharmaceutical, Takeda Pharmaceutical, AbbVie, EA Pharma, and Ferring Pharmaceuticals. Nagahori M has received consultancy fees from Takeda Pharmaceutical; and payment for lectures including service on speakers bureaus from Kissei Pharmaceutical, and Takeda Pharmaceutical, Kyorin Pharmaceutical, Mochida Pharmaceutical, AbbVie, Mitsubishi Tanabe Pharma, Nippon Kayaku, Asahi Kasei Medical, Zeria Pharmaceutical, Astellas, Nichi-Iko, and Janssen. Watanabe M has received payment for lectures including service on speakers bureaus from Mitsubishi Tanabe Pharma, Takeda Pharmaceutical, EA Pharma, Zeria Pharmaceutical, Janssen, Gilead Sciences, Celltrion Healthcare, and Pfizer; and grants from EA Pharma, Zeria Pharmaceutical, Mitsubishi Tanabe Pharma, Takeda Pharmaceutical, Nippon Kayaku, Mochida, Kissei, Miyarisan, Asahi Kasei, JIMRO, Kyorin Pharmaceutical, AbbVie, Kyowa Hakko Kirin, Alfresa, Ayumi, Astellas, MSD, Daiichi Sankyo, Taiho, Toray, Chugai, Gilead Sciences, and Fujirebio. Shikamura M, Yamaguchi T, Hori T, and Pinton P are employed by Takeda Pharmaceutical. Inaba T has nothing to declare.
Hibi T and Watanabe M are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Conception and design, data analysis and interpretation, and manuscript drafting and writing: Kobayashi T, Shikamura M, Yamaguchi T, Hori T, Pinton P. Data acquisition and manuscript writing: Ito H, Ashida T, Yokoyama T, Nagahori M, Inaba T, Watanabe M, Hibi T. All authors had access to the study data, reviewed and approved the final version of this manuscript, and agree to be accountable for all aspects of the work, which includes ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Others
The authors are grateful to the patients who participated in the trial and their families, investigators and the staff at the following study sites in Japan who supported this study: Ehime Prefectural Central Hospital; Fukuoka University Chikushi Hospital; Fukuoka University Hospital; Hamamatsu Minami Hospital; Hiroshima University Hospital; Infusion Clinic; Kagawa Prefectural Central Hospital; Keio University Hospital; Kitasato University Kitasato Institute Hospital; Kurume University Hospital; Kyorin University Hospital; Ofuna Chuo Hospital; Okayama University Hospital; Osaka City General Hospital; Saga University Hospital; Sapporo Higashi Tokushukai Hospital; Sapporo Kosei General Hospital; Sapporo Tokushukai Hospital; Shiga University of Medical Science Hospital; The Jikei University Hospital; Toho University Medical Center, Sakura Hospital; Tokyo Medical And Dental University; Tokyo Yamate Medical Center; Wakayama Medical University Hospital and Yokoyama IBD Clinic. The authors acknowledge the contribution of Eri Udagawa to development of this manuscript. Medical writing support was provided by Nicholas Crabb, MSc, of FireKite, an Ashfield company, part of UDG Healthcare plc, during the development of this manuscript, which was funded by Takeda Pharmaceutical Company Limited in compliance with Good Publication Practice 3 ethical guidelines (Battisti WP, et al. Ann Intern Med 2015;163:461-464).
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
Supplementary Table 1.
Supplementary Table 3.
REFERENCES
Table 1.
| Characteristic | Placebo (n = 10) | Vedolizumab SC (n = 10) | Vedolizumab IV (n = 2) |
|---|---|---|---|
| Age (yr) | 43.6 ± 13.0 | 46.0 ± 15.5 | 54.5 ± 19.1 |
| Male sex | 6 (60.0) | 7 (70.0) | 2 (100.0) |
| Weight (kg) | 58.2 ± 10.6 | 62.9 ± 16.3 | 61.8 ± 6.8 |
| Smoking classification | |||
| Never smoked | 4 (40.0) | 4 (40.0) | 1 (50.0) |
| Current smoker | 0 | 2 (20.0) | 0 |
| Ex-smoker | 6 (60.0) | 4 (40.0) | 1 (50.0) |
| Duration of UC (yr) | 10.3 ± 6.6 | 7.1 ± 4.0 | 8.2 ± 8.0 |
| Prior TNF-α antagonist therapy | 2 (20.0) | 5 (50.0) | 2 (100.0) |
| Concomitant immunomodulator use only at wk 0 | 1 (10.0) | 0 | 0 |
| Concomitant oral corticosteroid use only at wk 0 | 3 (30.0) | 3 (30.0) | 0 |
| Concomitant immunomodulator and corticosteroid use at wk 0 | 6 (60.0) | 7 (70.0) | 2 (100.0) |
| Disease localization | |||
| Left-sided colitis | 3 (30.0) | 4 (40.0) | 1 (50.0) |
| Pancolitis | 6 (60.0) | 5 (50.0) | 1 (50.0) |
| Fecal calprotectin (µg/g)a | |||
| ≤ 250 | 1 (10.0) | 0 | 0 |
| > 250 to ≤ 500 | 1 (10.0) | 0 | 0 |
| > 500 | 8 (80.0) | 10 (100.0) | 2 (100.0) |
| Mayo score | |||
| Mild (Mayo score < 6) | 0 | 0 | 0 |
| Moderate (Mayo score 6–8) | 4 (40.0) | 0 | 0 |
| Severe (Mayo score 9–12) | 6 (60.0) | 10 (100.0) | 2 (100.0) |
| Extraintestinal manifestation (yes) | 0 | 3 (30.0) | 0 |
Table 2.
| Outcome |
Placebo (n=10) |
Vedolizumab SC (n=10) |
Vedolizumab IV (n=2) |
|||
|---|---|---|---|---|---|---|
| No. (%) | 95% CI | No. (%) | 95% CI | No. (%) | 95% CI | |
| Clinical remission at week 52 | ||||||
| Yes | 2 (20.0) | (2.5, 55.6) | 4 (40.0) | (12.2, 73.8) | 1 (50.0) | (1.3, 98.7) |
| No | 8 (80.0) | (44.4, 97.5) | 6 (60.0) | (26.2, 87.8) | 1 (50.0) | (1.3, 98.7) |
| Vedolizumab vs. placebo | - | - | 20.0a | (–27.9, 61.8) | 30.0a | (–52.5, 86.0) |
| Endoscopic improvement at week 6 | ||||||
| Yes | 6 (60.0) | (26.2, 87.8) | 4 (40.0) | (12.2, 73.8) | 0 | |
| No | 4 (40.0) | (12.2, 73.8) | 6 (60.0) | (26.2, 87.8) | 2 (100.0) | (15.8, 100.0) |
| Endoscopic improvement at week 52 | ||||||
| Yes | 2 (20.0) | (2.5, 55.6) | 4 (40.0) | (12.2, 73.8) | 1 (50.0) | (1.3, 98.7) |
| No | 8 (80.0) | (44.4, 97.5) | 6 (60.0) | (26.2, 87.8) | 1 (50.0) | (1.3, 98.7) |
| Vedolizumab vs. placebo | - | - | 20.0a | (–27.9, 61.8) | 30.0a | (–52.5, 86.0) |
| Durable clinical response at week 6 and week 52 | ||||||
| Yes | 2 (20.0) | (2.5, 55.6) | 8 (80.0) | (44.4, 97.5) | 1 (50.0) | (1.3, 98.7) |
| No | 8 (80.0) | (44.4, 97.5) | 2 (20.0) | (2.5, 55.6) | 1 (50.0) | (1.3, 98.7) |
| Vedolizumab vs. placebo | - | - | 60.0a | (12.7, 88.5) | 30.0a | (–52.5, 86.0) |
| Durable clinical remission at week 6 and week 52 | ||||||
| Yes | 1 (10.0) | (0.3, 44.5) | 2 (20.0) | (2.5, 55.6) | 0 | |
| No | 9 (90.0) | (55.5, 99.7) | 8 (80.0) | (44.4, 97.5) | 2 (100.0) | (15.8, 100.0) |
| Vedolizumab vs. placebo | - | - | 10.0a | (–36.9, 53.9) | –10.0a | (–84.2, 71.0) |
| Corticosteroid-free remission at week 52 | ||||||
| No. of patients | 3 | 4 | 0 | |||
| Yes | 1 (33.3) | (0.8, 90.6) | 1 (25.0) | (0.6, 80.6) | 0 | |
| No | 2 (66.7) | (9.4, 99.2) | 3 (75.0) | (19.4, 99.4) | 0 | |
Table 3.
| Prior TNF-α antagonist therapy |
Placebo (n=10) |
Vedolizumab SC (n=10) |
Vedolizumab IV (n=2) |
|||
|---|---|---|---|---|---|---|
| No. (%) | 95% CI | No. (%) | 95% CI | No. (%) | 95% CI | |
| Prior TNF-α antagonist therapy | 2 | 5 | 2 | |||
| Yes | 0 | 1 (20.0) | (0.5, 71.6) | 1 (50.0) | (1.3, 98.7) | |
| No | 2 (100.0) | (15.8, 100.0) | 4 (80.0) | (28.4, 99.5) | 1 (50.0) | (1.3, 98.7) |
| TNF-α antagonist therapy naïve | 8 | 5 | 0 | |||
| Yes | 2 (25.0) | (3.2, 65.1) | 3 (60.0) | (14.7, 94.7) | 0 | |
| No | 6 (75.0) | (34.9, 96.8) | 2 (40.0) | (5.3, 85.3) | 0 | |
| Vedolizumab vs. placebo | - | - | 35.0a | (–23.4, 78.9) | - | - |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download