Abstract
Background/Aims
Methods
Results
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Hisamatsu T has received grants and personal fees from Mitsubishi Tanabe Pharma Corporation, EA Pharma Co. Ltd., AbbVie GK, JIMRO Co. Ltd., Zeria Pharmaceutical Co. Ltd., Kyorin Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Pfizer Japan Inc., and Mochida Pharmaceutical Co. Ltd.; grants from Asahi Kasei Kuraray Medical Co. Ltd., Daiichi Sankyo Co. Ltd., Nippon Kayaku Co. Ltd., and Astellas Pharma Inc.; and personal fees from Celgene, Janssen Pharmaceutical K.K., and Nichi-Iko Pharmaceutical Co. Ltd.
Suzuki Y has received honoraria from Takeda Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, AbbVie GK, EA Pharma Co. Ltd., Zeria Pharmaceutical Co. Ltd., Mochida Pharmaceutical Co. Ltd., and Kyorin Pharmaceutical Co. Ltd. and scholarship grants from Mitsubishi Tanabe Pharma Corporation, AbbVie GK, EA Pharma Co. Ltd., JIMRO Co. Ltd., Mochida Pharmaceutical Co. Ltd., Nippon Kayaku Co. Ltd., and Kissei Pharmaceutical Co. Ltd.
Kobayashi M, Hagiwara T, and Kawaberi T are employees of AbbVie GK.
Ogata H has received honoraria from Takeda Pharmaceutical Co. Ltd. and scholarship grants from Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co. Ltd., and Pfizer Japan Inc.
Matsui T has received honoraria from EA Pharma Co. Ltd., Ajinomoto Seiyaku, AbbVie GK, Eisai Co. Ltd., Kyorin Pharmaceutical Co. Ltd., Zeria Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co. Ltd., and Janssen Pharmaceutical K.K.; research grants from Takeda Pharmaceutical Co. Ltd., Miyarisan Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, and Otsuka Pharmaceutical Co. Ltd.; scholarship grants from AbbVie GK, Asahi Kasei Medical Co. Ltd., Astellas Pharma Inc., AstraZeneca K.K., EA Pharma Co. Ltd., Eisai Co. Ltd., MSD K.K., Otsuka Pharmaceutical Co. Ltd., JIMRO Co. Ltd., Zeria Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Takeda Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Nippon Kayaku Co. Ltd., and Mochida Pharmaceutical Co. Ltd.; and was a recipient of endowed chairs funded by AbbVie GK, Asahi Kasei Medical Co. Ltd., Ajinomoto Seiyaku, Inflammatory Bowel Disease Advanced Clinical Treatment, Regional/Emergency Medical Management (Fukuoka), Eisai Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Kyowa Hakko Kirin Co. Ltd., Kyorin Pharmaceutical Co. Ltd., JIMRO Co. Ltd., Zeria Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, UCB Japan Co. Ltd., EA Pharma Co. Ltd., Mochida Pharmaceutical Co. Ltd., and Chugai Pharmaceutical Co. Ltd.
Watanabe M has received honoraria from Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co. Ltd., EA Pharma Co. Ltd., Zeria Pharmaceutical Co. Ltd., Ajinomoto Seiyaku, and Eisai Co. Ltd.; research grants from Kaken Pharmaceutical Co. Ltd., Alfresa Pharma Corporation, and Eisai Co. Ltd.; scholarship grants from EA Pharma Co. Ltd., Zeria Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., MSD K.K., Daiichi Sankyo Co. Ltd., Taiho Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Nippon Kayaku Co. Ltd., Mochida Pharmaceutical Co. Ltd., Ayumi Pharmaceutical Corporation, Kissei Pharmaceutical Co. Ltd., and Miyarisan Pharmaceutical Co. Ltd.; and was a recipient of endowed chairs funded by Asahi Kasei Medical Co. Ltd., EA Pharma Co. Ltd., JIMRO Co. Ltd., Zeria Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Kyorin Pharmaceutical Co. Ltd., AbbVie GK, Kyowa Hakko Kirin Co. Ltd., MSD K.K., Toray Industries, Inc., Chugai Pharmaceutical Co. Ltd., Gilead Sciences, Inc., and Fujirebio Inc.
Hibi T has received lecture fees from Aspen Japan K.K, AbbVie GK, Ferring Pharmaceuticals Co. Ltd., Gilead Sciences, Inc., Janssen Pharmaceutical K.K., JIMRO Co. Ltd., Kissei Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co. Ltd., Nippon Kayaku Co. Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co. Ltd., and Zeria Pharmaceutical Co. Ltd.; advisory/consultancy fees from AbbVie GK, Bristol-Myers Squibb Company, Celltrion, EA Pharma Co. Ltd., Eli Lilly Japan K.K., Gilead Sciences, Inc., Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Nichi-Iko Pharmaceutical Co. Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Co. Ltd., and Zeria Pharmaceutical Co. Ltd.; research grants from AbbVie GK, EA Pharma Co. Ltd., JIMRO Co. Ltd., Otsuka Holdings Co. Ltd., and Zeria Pharmaceutical Co. Ltd.
Watanabe M and Hibi T are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Conceptualization: Hisamatsu T, Kobayashi M, Hagiwara T. Methodology: Kobayashi M, Hagiwara T, Kawaberi T. Formal analysis: Kobayashi M, Hagiwara T, Kawaberi T. Supervision: Hisamatsu T, Hibi T. Writing - original draft: Kobayashi M. Writing - review and editing: Hisamatsu T, Suzuki Y, Kobayashi M, Hagiwara T, Kawaberi T, Ogata H, Matsui T, Watanabe M, Hibi T. Approval of final manuscript: all authors.
Others
The standard operation procedure manual for data management and transformation was developed by A2 Healthcare Corporation (Tokyo, Japan) and approved by the Postmarketing Surveillance group at AbbVie GK (Tokyo, Japan). This support was funded by the sponsors (AbbVie GK and Eisai Co., Ltd.). AbbVie GK participated in the study design; data collection; analysis and interpretation of data; and writing, reviewing, and approval of the publication. Medical writing support was provided by Avinash Thakur (Mumbai, India) and Nicola West (London, UK) of Cactus Life Sciences (a part of Cactus Communications) and funded by the sponsors.
The authors would like to thank Kazuo Morita and Kaori Hozumi, employees of AbbVie, for assistance.
REFERENCES
Fig. 1.
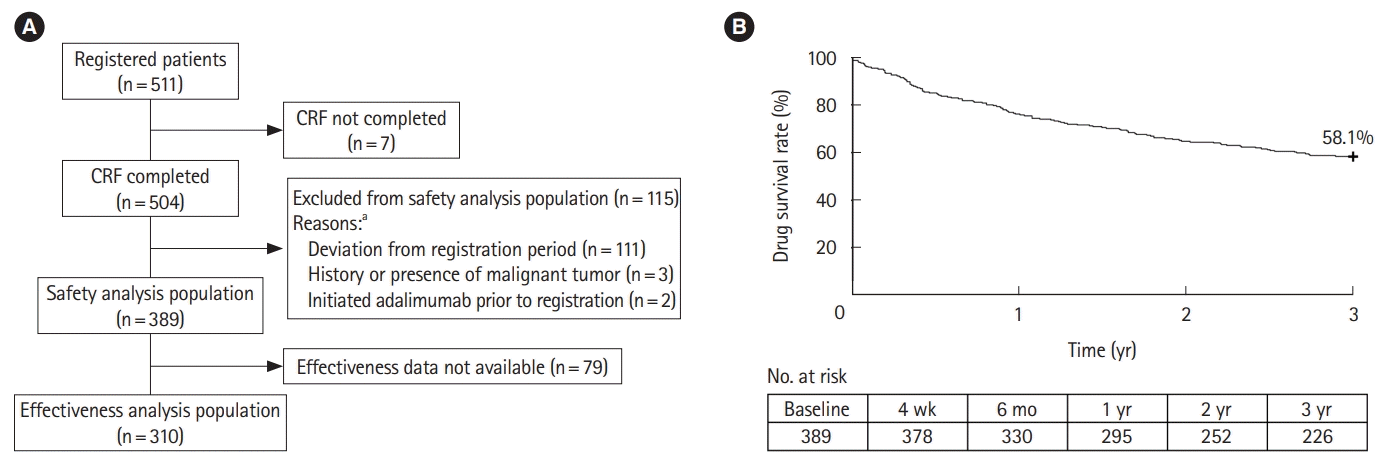
Fig. 2.
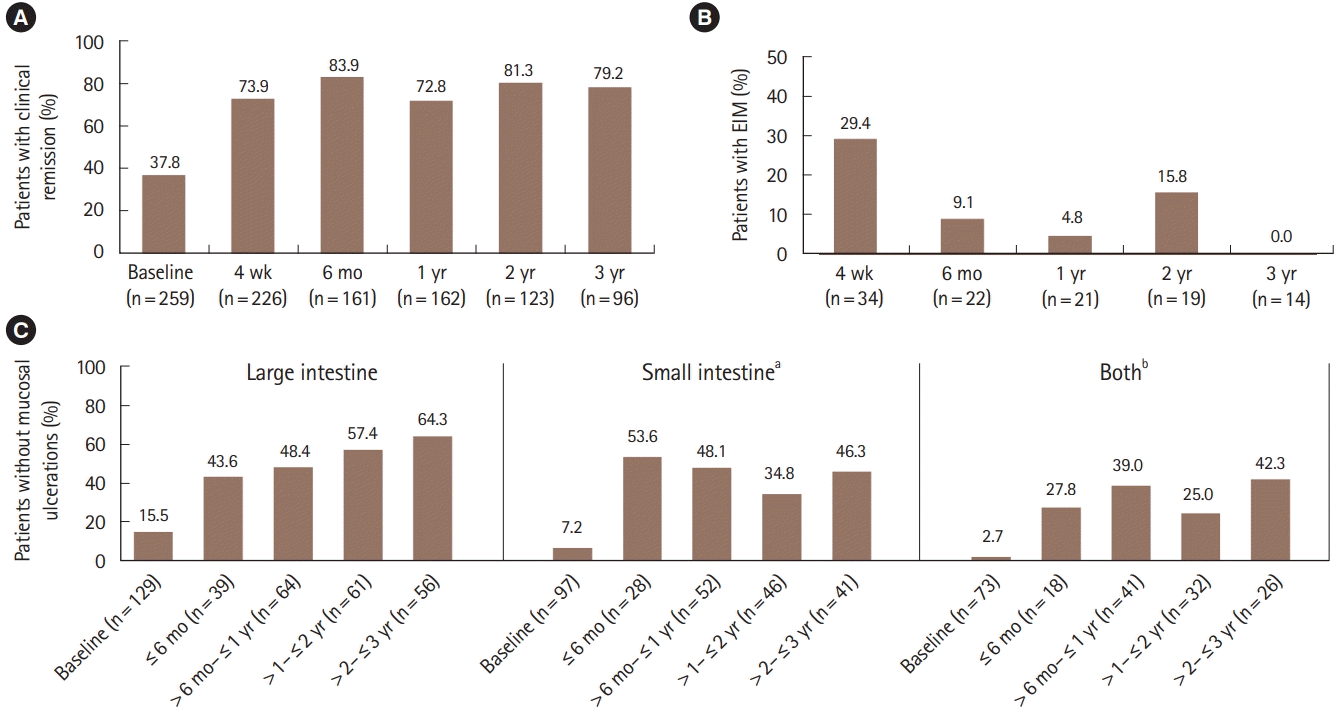
Fig. 4.
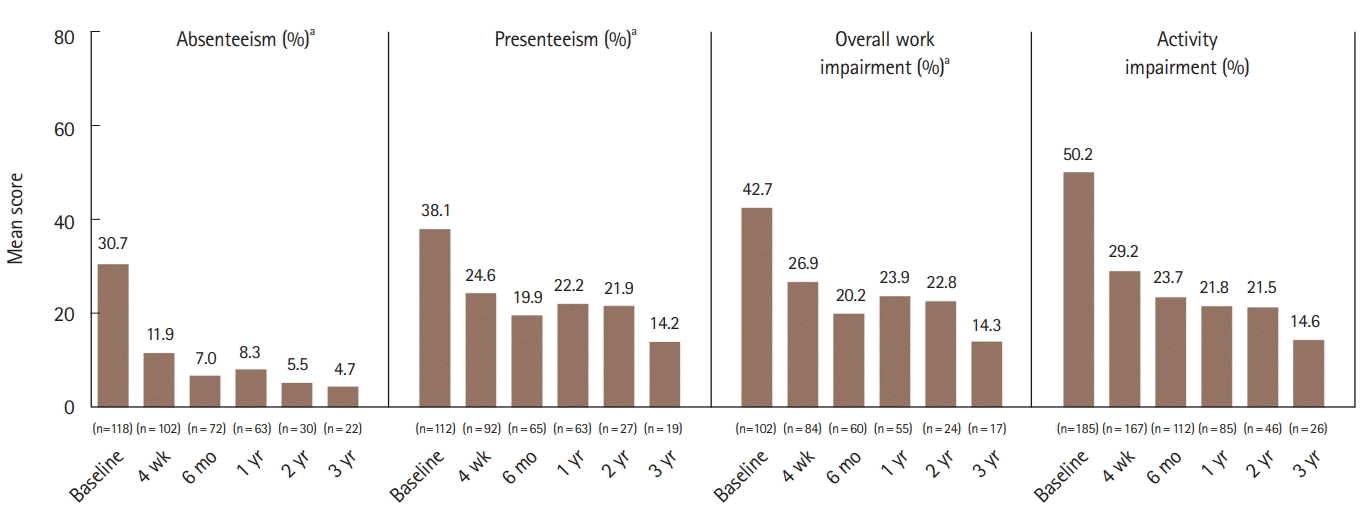
Table 1.
| Characteristic | Safety analysis population (n = 389) |
|---|---|
| Age (yr) | 34.0 ± 11.8 |
| Male sex | 283 (72.8) |
| Weight (kg) | 55.1 ± 10.4 |
| Duration of CD (yr) | 9.0 ± 9.4 |
| < 2 | 115 (29.6) |
| 2 to < 5 | 48 (12.3) |
| 5 to < 10 | 55 (14.1) |
| ≥ 10 | 142 (36.5) |
| Unknown | 29 (7.5) |
| History of smoking, never smoked | 243 (62.5) |
| Comorbidities | |
| Liver disorders | 17 (4.4) |
| Renal disorders | 5 (1.3) |
| Blood disorders | 44 (11.3) |
| Respiratory disorders | 11 (2.8) |
| Others | 71 (18.3) |
| CD-related pretreatment drugs | |
| Anti-TNF-α agent | 140 (36.0) |
| Aminosalicylates | 351 (90.2) |
| Corticosteroids | 62 (15.9) |
| Immunomodulators | 80 (20.6) |
| Antibiotics | 46 (11.8) |
| CD-related concomitant drugs | |
| Aminosalicylates | 336 (86.4) |
| Corticosteroids | 59 (15.2) |
| Immunomodulators | 125 (32.1) |
| Antibiotics | 49 (12.6) |
| Others | 286 (73.5) |
| Disease locationa | |
| Ileal | 104 (26.7) |
| Colonic | 57 (14.7) |
| Ileocolonic | 220 (56.6) |
| Isolated upper disease | 53 (13.6) |
| CD-related intestinal complications | 167 (42.9) |
| CD-related extraintestinal manifestations | 113 (29.0) |
| Self-administration of adalimumab | 342 (87.9) |
| Work status | |
| Employed ≥ 35 hr/wk | 192 (49.4) |
| Employed < 35 hr/wk | 32 (8.2) |
| Unemployed, but can perform normal daily activitiesa | 113 (29.0) |
| No normal daily activities (due to hospitalization, etc.) | 49 (12.6) |
| Unknown | 3 (0.8) |
| CDAI scorec | 193.9 ± 102.2 |
| < 150 | 101 (26.0) |
| 150 to < 220 | 65 (16.7) |
| 220 to < 450 | 98 (25.2) |
| ≥ 450 | 5 (1.3) |
| Unknown | 120 (30.8) |
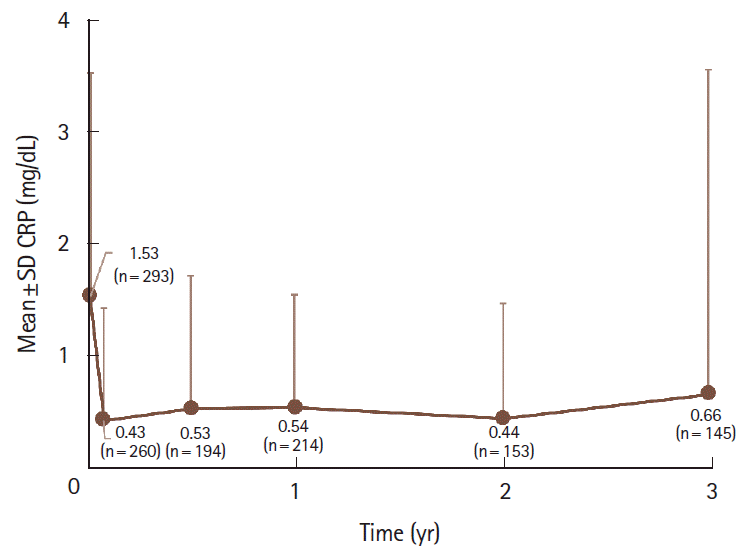
| CRP levels (mg/dL)d | 0.72 (0.19-2.14) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download