Abstract
Background/Aims
5-Aminosalicylic acid (5-ASA) is a basic drug for inducing and maintaining remission for ulcerative colitis. One of its formulations has a coating with a pH-dependent degradation that ensures the release 5-ASA at the terminal ileum. No evidence has been shown concerning the effects of proton pump inhibitors (PPIs) or H2 receptor antagonists (H2RAs) on the clinical course of ulcerative colitis patients in remission. The present study assessed the effect of PPIs or H2RAs on the relapse of ulcerative colitis patients in clinical remission maintained by pH-dependent released 5-ASA.
Methods
Ulcerative colitis patients who had been prescribed time- or pH-dependent-released 5-ASA between January 2015 and December 2018 were enrolled in this multicenter retrospective study. The period of remission until relapse occurred was analyzed among the patients taking time-dependent-released 5-ASA or pH-dependent-released 5-ASA with/without PPIs or H2RAs.
Results
One hundred and nineteen patients were analyzed in this study. In the primary endpoint, the relapse rate was higher in patients taking pH-dependent-released 5-ASA and PPIs or H2RAs than in those taking the pH-dependent-released 5-ASA without PPIs or H2RAs, while the relapse rate was similar in patients taking the time-dependent-released 5-ASA with or without PPIs or H2RAs concomitantly. Patients with a short duration of disease and middle-aged patients more frequently showed relapse with PPIs or H2RAs than the other patients.
Ulcerative colitis (UC) is a chronic relapsing inflammatory disease mainly affecting the large bowel mucosa. The prevalence of UC is high in Western countries and the number of UC patients is increasing in Westernized countries such as Japan as well as in many other Westernizing countries [1].
Most of the disease activity in UC patients is mild to moderate, and 5-aminosalicylic acid (5-ASA) has been the most basic drug administered to mild and moderate UC patients. Multiple studies have demonstrated the efficacy of 5-ASA for preventing relapse as well as inducing remission [2,3]. However, most of patients experience disease relapse, even when they continuously take 5-ASA. Once relapse occurs, patients need to take other medications, such as steroids, other immune modifying drugs or biologics [4]. Therefore, optimizing the use of 5-ASA is needed in order to ensure a better course in UC patients, including the longer maintenance of remission, an increase in the Inflammatory Bowel Disease Questionnaire, and a reduction in medication costs [5,6]. The ideal use of 5-ASA thus merits further investigation.
Clinicians use 5-ASA drugs such as Pentasa and Asacol, as authorized by the government of their countries. Due to the topical action of 5-ASA on colonic mucosa [7], formulations have been developed to deliver 5-ASA to the colonic mucosa with less systemic absorption. Pentasa has an ethylcellulose-based coating that starts to collapse when in contact with fluid, so it is the controlled-release formulation of 5-ASA designed to release therapeutic quantities of 5-ASA throughout the gastrointestinal tract in a time-dependent manner. In contrast, Asacol is coated with an acrylic-based resin, Eudragit S (methacrylic acid copolymer B, NF), which dissolves at a pH of 7 or greater [8], releasing 5-ASA in the terminal ileum and beyond to exert for topical anti-inflammatory action in the colon.
In the stomach, the pH is usually maintained under 3 by gastric acid [9,10]. Since peptic liquid from the pancreas and liver are alkaline, the pH increases in the intestine to 5–6 in the duodenum [11]. Proton pump inhibitors (PPIs) inhibit the H+-, K+- ATPase at the gastric parietal cells, while H2 receptor antagonists (H2RAs) block the action of histamine at the H2 receptors on the gastric parietal cells. Both drugs thus strongly inhibit the secretion of H+ and are effective for treating peptic ulcers. pH levels are maintained at 4–5 with PPIs and 3–4 with H2RAs in the stomach [12].
The pharmacokinetics of pH-dependent formulations were not shown to be affected by omeprazole coadministration for urinary and fecal excretion [13], nor were they affected by administration with famotidine [14]; however, the clinical data of UC patients concerning the coadministration of pH-dependent-released 5-ASA and acid-reducing agents have not been reported. We hypothesized that acid-reducing drugs increase the pH in the intestine and thus affect the 5-ASA release, especially for pH-dependent formulations, resulting in the relapse of disease. The present study assessed relapse in remission UC patients administered 5-ASA with or without the coadministration of acid-reducing agents.
For this retrospective observational cohort study, UC patients who were prescribed Pentasa or Asacol at the Department of Gastroenterology in Akita University Hospital or Omagari Kosei Medical Center between January 2015 and December 2018 were enrolled. Patients’ medical information and data were collected from electric medical records at each hospital. The collected information included the age, gender, disease duration, disease location, Mayo Endoscopic Score at all endoscopic examinations, partial Mayo score, period and dosage of prescribed Pentasa or Asacol, period of prescribed PPIs or H2RAs, prednisolone, azathioprine and biologics. Data were collected for all periods available in which patients were taking 5-ASA.
The inclusion criteria for this study were (1) a confirmed clinical, endoscopic and/or histological diagnosis of UC and (2) age between 15 and 65 years old in October 2019. The exclusion criteria were (1) patients who had a history of hypersensitivity or adverse events in response to 5-ASA, (2) patients who were receiving maintenance therapy with biologics, (3) period in which patients were receiving steroids, (4) patients who opted out of the study, and (5) patients with chronic continuous-type UC.
The primary objective of this study was to determine the risk of clinical relapse in UC patients administered the pH-dependent-released 5-ASA together with acid-reducing agents. Clinical remission was defined as a Mayo score ≤ 2 with a Mayo endoscopic score ≤ 1. Disease relapse was defined by an increase of 2 points in the Mayo score and the start of induction therapy by physicians. Periods in which the patients were prescribed Pentasa or Asacol and PPIs or H2RAs were analyzed. The non-disease-relapse period of the concomitant prescription of PPIs or H2RAs with 5-ASA was assessed with a Kaplan-Meier curve. Periods with an active phase for UC and with the administration of steroids for UC were not included in the analysis.
The results were represented as the means with the standard deviation. Background data were analyzed by Student t-test for paired variables or the chi-square or Fisher exact test for categorical variables. Kaplan-Meier curves were developed to examine the unadjusted relationship of the administration of acid-reducing agents with clinical relapse, with statistical comparisons analyzed using the log-rank test. Statistical analyses were performed using the GraphPad Prism8 software program (GraphPad Software, San Diego, CA, USA). A P-value < 0.05 was considered to be significant.
The protocol for this study was examined and approved by the Akita University School of the Medicine Ethics Committee (IRB No. 2381) and the Omagari Kosei Medical Center Ethics Committee (IRB No. 19-032). The study design was published, and patients had a chance to opt out of the use of their information for this study.
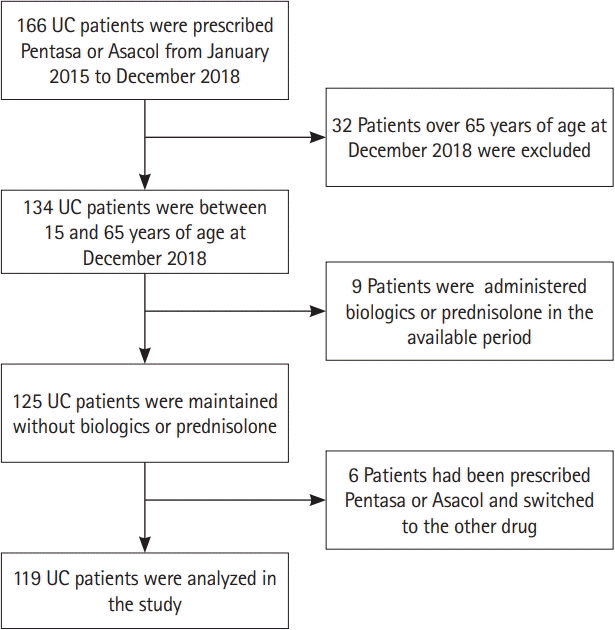
Among the 166 UC patients prescribed Pentasa or Asacol from January 2015 to December 2018, 32 were excluded because of their age, and 9 were excluded because they had been administered biologics or prednisolone concomitantly. Six patients were prescribed either Pentasa or Asacol and switched to the other drug. One hundred and nineteen patients were thus included in the analysis (Fig. 1). Sixty-one patients were prescribed with Pentasa and 64 patients were prescribed with Asacol. We confirmed that all patients took medicine with over 90% adherence on the medical record. The patients’ characteristics are shown in Table 1. The duration of disease was longer (P= 0.001), and the age tended to be older (P= 0.051) in the patients who were taking Pentasa than in those who were taking Asacol. Pentasa was approved for the treatment of UC earlier than Asacol in Japan (1996 and 2009, respectively). In the present populations, PPIs and H2RAs were prescribed for functional dyspepsia or prescribed for preventing antidrug inducing peptic ulcers with nonsteroidal anti-inflammatory drugs (NSAIDs).
In the primary endpoint, the relapse rate was 85.1% and 86.9%, 74.5% and 84.5% in Pentasa and Pentasa with anti-acid agents in 1 year, and 2 years, respectively. On the other hand, the relapse rate was 87.3% and 70.7%, 70.5% and 46.1% in Asacol and Asacol with anti-acid agents in 1 year, and 2 years, respectively. Importantly, the relapse rate was higher in patients taking Asacol and acid-reducing agents than that in those taking Asacol without acid-reducing agents (P= 0.04), while the relapse rate was similar in patients taking Pentasa with or without acid-reducing agents concomitantly (P= 0.5) (Fig. 2).
Subpopulations in the Pentasa and Asacol groups who were or were not taking acid-reducing agents are shown in Table 2. The duration of disease tended to be longer in patients taking Pentasa with acid-releasing agents (P= 0.08) and Asacol with acid-reducing agents (P= 0.07) than in those not taking acidreducing agents, and proctitis was tended to be more frequent (P= 0.10) in patients taking Asacol without acid-reducing agents but not to a significant degree.
The periods available in this analysis are shown in Table 3. There were no marked differences in azathioprine use for maintenance therapy among the patients who were or were not taking acid-reducing agents in each group. A stratified subanalysis was performed for the gender, age and disease duration. Ten or fewer years’ duration (P= 0.049) and an age of 50 to 65 years old (P= 0.01) were factors significantly associated with relapse while taking acid-reducing agents, whereas more than 10 years’ duration, under age of 50, and the gender had no influence (Fig. 3).
No study has described the induction of relapse by acid-reducing agents in remission UC patients maintained by 5-ASA. Hussain et al. [13] reported that omeprazole did not influence the urinary or fecal excretion of pH-dependent-released 5-ASA in healthy subjects. However, our study showed that co-treatment with the acid-reducing agent Asacol clearly affected the clinical course in UC. Such a situation is not rare, as acid-reducing agents are usually prescribed for functional dyspepsia, which is quite common, and UC patients are no exception [15].
Our data also showed that middle-aged patients were more often affected by administration of Asacol than younger patients. We did not assess the fecal excretion and mucosal concentration with 5-ASA. However, the possible explanation for this result is due to differences in the rate of Helicobacter pylori infection and the relative infrequency of bowel movements in older persons. Previous studies showed that baseline gastric acid secretion is decreased [16,17] and the anti-secretory effects of PPIs more pronounced [9,10] in H. pylori-positive subjects than in H. pylori-negative ones. We excluded patients over 65 years of age in order to assess the effects of acid-reducing agents more accurately, as this age group is markedly influenced by H. pylori infection, which affects the acid production in the stomach [16,17]. Recently, the number of older UC patients has been increasing. These older patients are often taking multiple medicines, including acid-reducing agents for gastritis or functional dyspepsia or even preventing ulcers with NSAIDs or aspirin. We should bear these results in mind in order to avoid the long-term prescription of acid-reducing agents together with pH-dependent-released 5-ASA. Our data also demonstrated a short duration showed more frequent relapse in concomitant administration of acid-reducing drug with Asacol. Previous study showed that long duration of disease was protective against the relapse [18]. Based on this result, one possible explanation is that the effect of acid-reducing agents concomitant with Asacol was enough to lead to relapse for the patients with short duration but not for the patients with long duration.
Chronic atrophic gastritis caused by H. pylori infection leads to less production of gastric acid and a subsequent increase in the pH in the stomach (to 3-4) [17,19]. Because the duodenal fluid is alkaline due to pancreatic and biliary acid, the pH in chronic atrophic gastritis patients increases all the way to 7 [11]; this results in the earlier degradation of the coating of Asacol in the small intestine of such patients than in healthy person. Basically, over half of the population ≥ 65 years old has gastric atrophy due to H. pylori infection in Japan [16,17]. In addition, given that the rate of H. pylori infection increases with age, one of the explanation of that middle-aged patients were more affected by the administration of acid-reducing agents than younger patients could be the H. pylori infection.
Limitation of this retrospective study is the bias from drugs being chosen by each physician, the collection bias due to not being able to obtain completely correct data retrospectively, the lack of data on topical medication or cytapheresis therapy, and the lack of data on atrophic gastritis. We did not assess adjusted risk of relapse with confounding factors such as smoking or NSAIDs use in this study [20]. It should be considered to analyze for further investigation in the future. We need to conduct a prospective study to further elucidate the effects of acid-reducing agents. This study design was designed to elucidate the effect of acid-reducing agents for maintenance of remission. Since 5-ASA is also a key drug leading to the induction of remission in UC, the effect of acid-reducing agents should be studied in the induction phase.
In conclusion, in this multicenter retrospective study, coadministration of PPIs or H2RAs affects relapse of UC in remission maintained by pH-dependent-released 5-ASA but not time-dependent-released 5-ASA. The data of subanalysis suggest UC patients with a short duration of disease and middle-aged patients are more affected by these acid-reducing agents.
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contribution
Conceptualization: Shimodaira Y. Data curation: Shimodaira Y, Onochi K, Watanabe K, Takahashi S, Fukuda S, Watanabe N, Koizumi S, Matsuhashi T. Formal analysis: Shimodaira Y. Methodology: Shimodaira Y. Project administration: Shimodaira Y. Supervision: Iijima K. Writing - original draft: Shimodaira Y. Writing - review & editing: Iijima K. Approval of final manuscript: all authors.
REFERENCES
1. Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017; 152:313–321.

2. Jeong DY, Kim S, Son MJ, et al. Induction and maintenance treatment of inflammatory bowel disease: a comprehensive review. Autoimmun Rev. 2019; 18:439–454.

3. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017; 389:1756–1770.

4. Burger D, Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology. 2011; 140:1827–1837.

5. Irvine EJ, Yeh CH, Ramsey D, Stirling AL, Higgins PD. The effect of mesalazine therapy on quality of life in patients with mildly and moderately active ulcerative colitis. Aliment Pharmacol Ther. 2008; 28:1278–1286.

6. Buckland A, Bodger K. The cost-utility of high dose oral mesalazine for moderately active ulcerative colitis. Aliment Pharmacol Ther. 2008; 28:1287–1296.

7. Frieri G, Giacomelli R, Pimpo M, et al. Mucosal 5-aminosalicylic acid concentration inversely correlates with severity of colonic inflammation in patients with ulcerative colitis. Gut. 2000; 47:410–414.

8. Abinusawa A, Tenjarla S. Release of 5-aminosalicylic acid (5-ASA) from mesalamine formulations at various pH levels. Adv Ther. 2015; 32:477–484.

9. Verdú EF, Armstrong D, Idström JP, et al. Effect of curing Helicobacter pylori infection on intragastric pH during treatment with omeprazole. Gut. 1995; 37:743–748.

10. Labenz J, Tillenburg B, Peitz U, et al. Helicobacter pylori augments the pH-increasing effect of omeprazole in patients with duodenal ulcer. Gastroenterology. 1996; 110:725–732.

11. Hamlet A, Olbe L. The influence of Helicobacter pylori infection on postprandial duodenal acid load and duodenal bulb pH in humans. Gastroenterology. 1996; 111:391–400.

12. Miner PB Jr, Allgood LD, Grender JM. Comparison of gastric pH with omeprazole magnesium 20.6 mg (Prilosec OTC) o.m. famotidine 10 mg (Pepcid AC) b.d. and famotidine 20 mg b.d. over 14 days of treatment. Aliment Pharmacol Ther. 2007; 25:103–109.

13. Hussain FN, Ajjan RA, Moustafa M, Weir NW, Riley SA. Mesalazine release from a pH dependent formulation: effects of omeprazole and lactulose co-administration. Br J Clin Pharmacol. 1998; 46:173–175.

14. Wiltink EH, Mulder CJ, Stolk LM, Rietbroek R, Verbeek C, Tytgat GN. Absorption of oral mesalazine-containing preparations and the influence of famotidine on the absorption. Scand J Gastroenterol. 1990; 25:579–584.

15. Barros LL, Farias AQ, Rezaie A. Gastrointestinal motility and absorptive disorders in patients with inflammatory bowel diseases: prevalence, diagnosis and treatment. World J Gastroenterol. 2019; 25:4414–4426.

16. Iijima K, Sekine H, Koike T, Imatani A, Ohara S, Shimosegawa T. Serum pepsinogen concentrations as a measure of gastric acid secretion in Helicobacter pylori-negative and -positive Japanese subjects. J Gastroenterol. 2005; 40:938–944.

17. Iijima K, Koike T, Abe Y, Ohara S, Nakaya N, Shimosegawa T. Time series analysis of gastric acid secretion over a 20-year period in normal Japanese men. J Gastroenterol. 2015; 50:853–861.

18. Bello C, Belaiche J, Louis E, Reenaers C. Evolution and predictive factors of relapse in ulcerative colitis patients treated with mesalazine after a first course of corticosteroids. J Crohns Colitis. 2011; 5:196–202.

Fig. 2.
Kaplan-Meier curves demonstrating the relapse-free rate in ulcerative colitis. Hashed lines indicate censored cases. (A) Patients prescribed Pentasa (thick line) and Pentasa with PPIs or H2RAs (solid line). (B) Patients prescribed Asacol (thick line) and Asacol with PPIs or H2RAs (solid line). PPIs, proton pump inhibitors; H2RAs, H2 receptor antagonists.
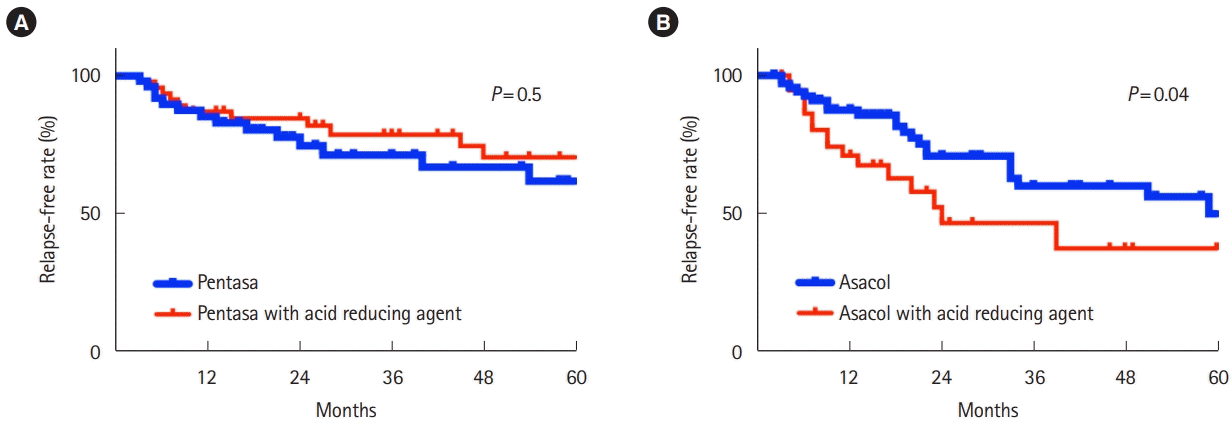
Fig. 3.
Kaplan-Meier curves demonstrating the relapse-free rate in ulcerative colitis. Hashed lines indicate censored cases. Patients prescribed Asacol (thick line) and Asacol with PPIs or H2RAs (solid line). Patients 15–49 years of age (A) and 50–65 years of age (B) were shown. Patients prescribed Asacol (thick line) and Asacol with PPIs or H2RAs (solid line). Patients of 10 or fewer years’ disease duration (C) and more than 10 years’ disease duration (D) were shown. PPIs, proton pump inhibitors; H2RAs, H2 receptor antagonists.
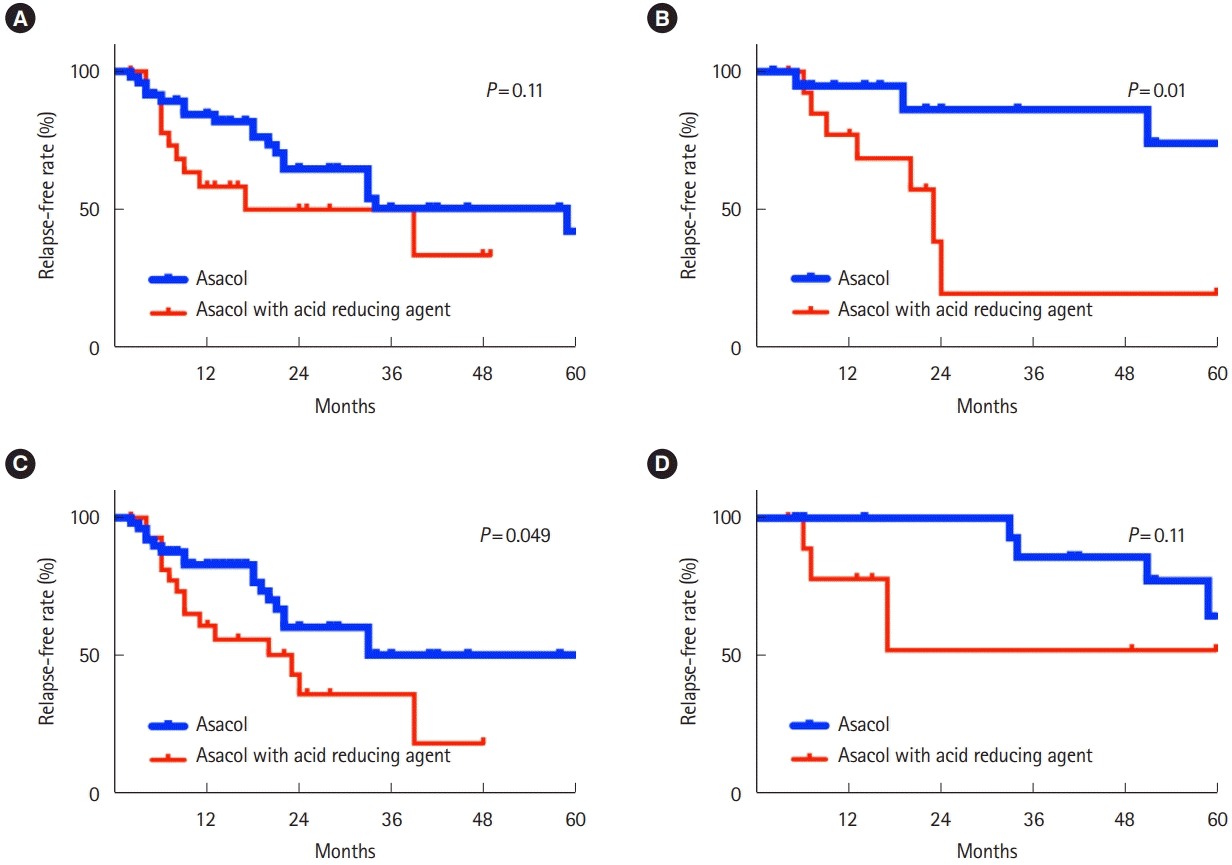
Table 1.
Characteristics of Included Patients in This Study
| Characteristics | Total (n = 119)a | Pentasa (n = 61) | Asacol (n = 64) | P-value |
|---|---|---|---|---|
| Sex | 0.210 | |||
| Male | 73 (61.3) | 32 (52.5) | 41 (64.1) | |
| Female | 46 (38.7) | 29 (47.5) | 23 (35.9) | |
| Age (yr) | 42.0 ± 14.4 | 44.8 ± 15.0 | 39.6 ± 13.4 | 0.051 |
| Duration of disease (yr) | 10.7 ± 8.0 | 13.9 ± 8.2 | 7.5 ± 6.3 | 0.001 |
| Disease location | 0.030 | |||
| Total | 61 (51.3) | 33 (54.1) | 31 (48.4) | |
| Left-sided | 38 (31.9) | 22 (36.1) | 17 (26.6) | |
| Proctitis | 11 (9.2) | 2 (3.3) | 11 (17.2) | |
| Dose (mg/day) | 2,885 ± 1,020 | 3,138 ± 675 | 0.010 | |
| Azathioprine | 28 (23.5) | 8 (13.1) | 22 (34.4) | 0.010 |
| PPIs | 32 (26.9) | 15 (24.6) | 19 (29.7) | 0.550 |
| Dosing period (mon) | 36.1 ± 30.0 | 26.7 ± 25.2 | ||
| H2RAs | 36 (30.3) | 25 (41.0) | 13 (20.3) | 0.020 |
| Dosing period (mon) | 76.8 ± 58.3 | 29.6 ± 20.3 | ||
| Biologics | 17 (14.3) | 9 (14.8) | 9 (14.1) | 1.000 |
Table 2.
Subpopulations in the Pentasa or Asacol Groups
Table 3.
Maintenance Drugs in Remission of Ulcerative Colitis




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download