Abstract
Background/Aims
Ulcerative colitis (UC) is a chronic inflammatory disorder with indefinite etiology; however, environmental, genetic, immune factors and microbial agents could be implicated in its pathogenesis. UC treatment is lifelong, therefore; the potential side effects and cost of the therapy are significant. Yarrow is a promising medicinal plant with the ability to treat many disorders, owing to its bioactive compounds especially the essential oil. The main aim of this research was to investigate the therapeutic effect of the yarrow oil on colitis including the involved mechanism of action.
Methods
In 21-female C57BL/6 mice were divided into 3 groups; control group, colitis model group, and oil-treated group. Groups 2 and 3 received 5% dextran sulfate sodium (DSS) in drinking water for 9 days, and concomitantly, only group 3 was given 100 mg/kg yarrow oil. Mice were examined for their body weight, stool consistency and bleeding, and the disease activity indexes were calculated.
Results
Oral administration of yarrow oil markedly repressed the severity of UC via the reduction of the inflammatory signs and restoring colon length. The oil was able to down-regulate nuclear factor kappa light chain enhancer of activated B cells (NF-κB), up-regulate peroxisome proliferator-activated receptor gamma (PPAR-γ), and enhance transforming growth factor-β expression. The oil normalized the tumor necrosis factor-α expression, restored the normal serum level of interleukin-10 (IL-10) and reduced the serum level of IL-6.
Ulcerative colitis (UC), a type of inflammatory bowel disease (IBD), is a chronic inflammatory disorder, affects both males and females equally [1]. Data analysis revealed that UC prevalence has slightly increased over time and reported in all decades [2]. The etiology of UC is unknown, however, the complicated interplay of environmental, genetic, immune factors and microbial agents are claimed to be involved [3], which enables luminal toxins, antigens, and pathogens to penetrate the mucosal layers and exaggerate the intestinal inflammation via provoking the pro-inflammatory cytokines and migration of effector leukocytes [4]. The main aim of the treatment is to reduce the colon inflammation and maintain remission without the need to escalate the treatment or add corticosteroids. In mild cases, the oral or rectal conventional therapy (sulfasalazine and amino-salicylates) is often enough to make remission, while in moderate cases, corticosteroids are used alone or with other classes of medications to achieve remission. On another hand, in some severe cases, conventional therapy is not effective but biologics (anti-tumor necrosis factor [TNF] and Janus kinase inhibitors) are required for maintenance of remission [5].
Yarrow (Achillea millefolium, family Asteraceae), known in the Middle East as “Alkaysoom Alfi Alawrak or Alhozonbol,” is one of the most commonly used herbs in the Middle East and the Arab World [6]. It is used in folk medicine as a treatment for many disorders related to the gastrointestinal (GI) tract, cardiovascular system, sleep inability, several types of inflammation and anxiety [7]. It is proven that the plant contains many components of medicinal value such as alkaloids [8], flavonoids [9], and volatile oil [10]. The essential oil of yarrow is one of the main constituents of plant [10]. The oil is characterized by a very deep blue color when extracted by hydrodistillation due to the presence of chamazulene, however, when the oil is extracted by other methods such as supercritical carbon dioxide extraction, the blue color becomes faint [11]. Yarrow oil ingredients vary depending on location, environment, and weather, however, the oil shows a consistent pattern of the main components such as sabinene, pinene, 1,8-cineole, borneol, β-caryophyllene, germacrene, and chamazulene [10-12], Yarrow essential oil is clinically recognized as a treatment for many diseases and syndromes such as wounds and other skin-inflammatory conditions [13,14], digestive system problems such as cramps and constipation [15], and circulatory disorders such as varicose veins and hemorrhoids [16].
The first strategy for the treatment of UC is the anti-inflammatory drugs, which are commonly used and are effective against UC. However, the potential side effects are important considerations since the treatment of UC requires a long time. The second strategy for the treatment of UC is biologics; however, this choice suffers from high cost especially for low-income populations. Hence, there is a need to find a new safe, cheap, and effective drugs to achieve clinical remission of UC. In this regard, the main objective of this study is to verify the efficacy of yarrow oil for the treatment of colitis and clarify its mechanism of action.
Dextran sulfate sodium (DSS) was obtained from MP Bio-medicals (Solon, OH, USA). Mouse interleukin-6 (IL-6) ELISA kit (ab 100712) and mouse IL-10 ELISA kit (ab 100697) were obtained from Abcam (Cambridge, UK). Nuclear factor kappa light chain enhancer of activated B cells (NF-kB; sc-372), peroxisome proliferator-activated receptor γ (PPAR-γ; sc-7273), TNF-α (sc-1351), transforming growth factor-β (TGF-β; sc28345) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; sc-32233), goat anti-rabbit immunoglobulin G (IgG)-horseradish peroxidase (HRP; sc-2030), goat anti-mouse IgG-HRP (sc-2005) antibodies, luminol reagent (sc-2048), and polyvinylidene fluoride (PVDF) membrane (sc-3723) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The remaining chemicals used were of analytical grade and obtained from a local distribution agent in Kingdom of Saudi Arabia.
The yarrow plant (Achillea millefolium L., family Asteraceae) was collected from local farms in diameter of 3 km in Al-Ahsa, Eastern Province, Kingdom of Saudi Arabia, in January 2018 after permissions from the farms’ owners. Samples are not likely to have a significant change in components or their percentages. Furthermore, all samples were collected together and only one oil sample was produced and used throughout all experiments. Dr. A.H. Abdel-Baset, plant taxonomist, The Egyptian Agricultural Museum, Cairo, Egypt, kindly identified the plant. Voucher specimens (No. AS143) were deposited in the herbarium of the College of Clinical Pharmacy, King Faisal University, Kingdom of Saudi Arabia.
The dried A. millefolium whole plant (100 g) was cut and subjected to hydrodistillation using Clevenger-type apparatus for 3 hours [17]. The volatile fraction (yield; 0.85% v/dried weight) was recovered by decantation and was dried over anhydrous sodium sulfate. The essential oil samples were kept in brown vials in the refrigerator at 4°C until further use.
The isolated yarrow essential oil was subjected to gas chromatography analysis. Gas chromatography/flame ionization analysis was accomplished using GC-2010 Plus (Shimadzu Corp., Kyoto, Japan) gas chromatograph outfitted with FID-2010 Plus detector. The column was RTX-5MS® fused silica capillary (30 m × 0.25 mm inner diameter and 0.25 μm film thickness); the oven temperature initially was held at 40°C for 1 minute and then increased to 240°C at 3°C/min. The carrier gas was helium with a flow rate of 1.0 mL/min; the temperature of injector and detector were 250°C and 300°C, respectively, the split ratio was 1:20 and the injection volume is 5 μL. Quantification of essential oil components was performed by relative parentage area calculations. The relative percentage of the essential oil constituents was assessed on basis of the FID responses from the total peak area using percentage area normalization [11,17].
Gas chromatography/mass spectrometry data were performed on GCMS-QP2010 Plus (Shimadzu Corp.). The ionization energy for the mass spectrometer was 70 eV. The other conditions were identical to those mentioned for gas chromatography/flame ionization. Kovats retention indices were calculated with respect to a set of co-injected standard hydrocarbons (C10-C28; Sigma Aldrich, Darmstadt, Germany). Compounds were identified by comparing their spectral data and retention indices with Wiley Registry of Mass Spectral Data 10th edition (April 2013), NIST 11 Mass Spectral Library (NIST11/2011/EPA/NIH) and literature data [17]. All the identified compounds and their percentages are listed in Tables 1 and 2.
Twenty-one 8-week-old female C57BL/6 mice average weight 20 ± 2.5 g were used in this study. The animals were obtained as a gift from Dr. Hamza Hanie, animal facility, College of Science, King Faisal University. Mice were housed in the air-conditioned animal house, a 12-hour light/12-hour dark cycle, adjusted at 22°C ± 1°C temperature. During the study periods, all animals were fed a standard rat diet.
The Animal Research Ethics Committee at King Faisal University (approval No. KFU-REC/2018-6-5) approved all animal experimental procedures and protocols and they were performed in accordance with the Guidelines for the Ethical Conduct for Use of Animals in Research, King Faisal University.
A preliminary study was done (results not shown) to investigate the therapeutic and toxic doses from yarrow oil on experimental mice, using concentration from 1, 5, 10, 20, 50, 100, 200 to 500 mg/kg. These preliminary studies are common practice before working with essential oil to determine the dose and to determine the toxicity. The least dose produced a therapeutic effect was 100 mg/kg. For toxicity studies, we treated normal control mice by doses of oil from 50, 100, 200, 500, and 1,000 mg/kg and no symptoms of toxicity were noticed. We used water as a vehicle since the oil is miscible with water.
Mice were divided into 3 groups, 7 mice in each group; healthy control group (control), which were given tap water, UC induced-group (DSS), which received 5% DSS freshly prepared in drinking water (DSS), and yarrow oil-treated group (oil), received 100 mg/kg of oil diluted with water. The oil was given once daily via gavage concomitant with the DSS-treatment period. The control group continued on tap water for drinking while both of DSS-group and oil-group were given 5% DSS in the drinking water for 9 days [18]. We used the well-established protocol in our laboratory on C57BL/6 mice to induce acute colitis using 5% DSS freshly prepared daily in distilled water as described in [18-20]. The key point in preparing the model is the monitoring signs of developing colitis by measuring the mice’s body weight and occult blood in the feces. Moreover, to prepare chronic colitis low concentration of DSS is used for a long time as we used in our previous publication [21].
During the period of the experiment, the animals were monitored for food intake, body weight, and fecal output. The disease activity was determined using the change in body weight, bleeding and occult blood as described elsewhere [22]. On day 9 all mice, that have manifested the criteria of UC, were sacrificed by cervical dislocation and colons were cut and their length was measured [23]. Part of colons was preserved in 10% buffered formalin for histopathology testing [24], and the remaining parts were rapidly homogenized on ice for chemical and immunoblotting studies.
Colitis was assessed using the disease activity index (DAI) as described in [25]. In brief, bodyweight loss, bleeding, and stool consistency were scored. Weight loss of 0, 1–5, 5–10, 10–20, and > 20% were scored as 0, 1, 2, 3, and 4, respectively. For stool consistency, 0 was scored for normal well-formed particles, 1 for loose stools, 2 for semi-formed stools, 3 for liquid stools, and 4 for diarrhea. Bleeding was scored 0 for no blood, 1 for trace, 2 for mild hemoccult, 3 for obvious hemoccult, and 4 for gross bleeding. The calculated sub-scores were summed and the total was divided by 3 to obtain the DAI scores, which ranges from 0 to 4.
Eight randomly selected fields were inspected in each section by histopathologist blinded to the treatment protocol. The grading scale of colonic inflammation was determined as shown in (Table 3), for each category as inflammation, destruction of crypt and depth of lesions. The sum scores of inflammation and depth of lesions were added to the inflammatory damage score (range, 0–24). The destruction score of the crypt represents the crypt damage score (range, 0–16) [25].
IL-6 and IL-10 were determined in serum using the kits described under the chemical and reagent section and according to manufacturer protocols. NF‐κB, PPAR-γ, TNF-α, TGF-β, and GAPDH expression were determined in colon homogenates by Western blot analysis. The blot analysis was performed as previously described [21]. Briefly, colon samples were homogenized in radio immunoprecipitation assay (RIPA) buffer with a protease inhibitor; the total protein extracted from those homogenates was quantified using a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Then, 50 μg of the total extracted protein was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and blotted on PVDF membranes. The membranes were incubated with the primary antibodies (1:300 dilutions) for 2 hours at room temperature, and they were then incubated with the secondary antibody (goat antirabbit HRP-conjugated at a 1:5,000 dilution). The chemiluminescence produced from the luminol reagent was detected with the LI-COR C-DiGit chemiluminescence scanner (Lincoln, NE, USA) and the intensity of the bands was analyzed using the scanner software. β-Actin was used as a protein loading control.
Data are presented as mean ± standard deviation from animals in each group and the difference was determined using Students t-test between the groups. P<0.05 was considered statistically significant. GraphPad Prism software version 4 (GraphPad Software, San Diego, CA, USA) was used for the analysis.
The yield of essential oil obtained from A. millefolium after hydrodistillation was 0.85% (± 0.02%) volume/dried weight (from 3 independent experiments). Overall, 41 components were separated from the essential oil after the gas chromatography analysis with 19 components being identified and quantified. The identified compounds were corresponding to 94.19% of the total separated components area percentage, and the chemical names of those 19 compounds are illustrated in Table 1.
For the 19 components, 14 are monoterpenes and 5 are sesquiterpenes, however, those 5 sesquiterpenes represent 50.93% of the total area percentage of the whole oil components. Germacrene D (26.15%), chamazulene (10.04%), and β-caryophyllene (10.35%), were quantified as the major sesquiterpene in the essential oil. In addition to sabinene (14.28%), β‐pinene (11.40%), and borneol (5.26%) were the major monoterpenes. 15.66% (7 compounds) of the essential oil components were oxygenated and nearly 50% (7.81%) of the oxygenated compound were alcohols, and the most abundant alcohol was borneol (5.26%). Yarrow essential oil contains many cyclization patterns, with the majority of compounds being bicyclic (10 compounds, 56.40%) and then monocyclic (7compounds, 32.31%). Two com-pounds, o-cymene (0.66%) and chamazulene (10.04%) contain aromatic cycles (Tables 1, 2).
Mice were treated as described in the experimental part to prepare the colitis model and the disease was manifested through the decrease of body weight, stool uniformity in addition to bleeding symptoms. The loss of body weight and unformed stool has been noticed from the 5th day and became obvious on the 9th day in the DSS-group. On the other hand, unformed stool in the oil-treated group was noticed on the 8th day. DSS-induced colitis group showed significantly higher DAI score compared to the control group (3.62 ± 0.037 vs. 0.35 ± 0.06, P<0.001). Compared with DSS-induced colitis, oil treatment resulted in a significant decrease in the DAI score (3.62 ± 0.037 vs. 1.50 ± 0.27, P<0.001) (Fig. 1A). DSS-induced significant colon reduction in comparison to the healthy control group (2.75 ± 0.25 cm vs. 4.88 ± 0.36 cm, P<0.001), while the reduction in colon length was less manifested in the oiltreated group and there was a significant difference in the colon length in comparison to the DSS group (3.37 ± 0.27 cm vs. 2.75 ± 0.25 cm, P<0.01) (Fig. 1B). Moreover, treatment with the oil improved the symptoms of colitis as noticed from abolishing of the redness and bleeding (Fig. 1C).
To define the mechanisms that may participate in the therapeutic effects of yarrow oil, we determined the expression of some pro- and anti-inflammatory cytokines in the colon tissues by Western blot (Fig. 2).
Mice with DSS-induced colitis exhibited statistically (P<0.001) increased expression of NF-κB and TNF-α compared with control mice. The expression of NF-κB was significantly (P<0.01) down-regulated by treatment with the yarrow oil while TNF-α expression was almost normalized (Fig. 2A and B). On the other hand, the expression of PPAR-γ was decreased significantly (P<0.001) in the DSS-induced colitis, and the yarrow oil significantly (P<0.001) enhanced its expression (Fig. 2C). The inflammatory process regulator, TGF-β protein, was massively produced with the administration of the yarrow oil. TGF-β was 3-fold more expressed in the oiltreated group than in the control group (Fig. 2D).
DSS-treated mice showed a significant decrease (P<0.001) in serum level of, the anti-inflammatory cytokine, IL-10, in comparison to the control group; however, the yarrow oil significantly enhanced its level (P<0.01) (Fig. 3A). The serum level of IL-6 in mice treated with DSS was significantly (P<0.001) increased in comparison with the control group. However, this increased level of serum IL-6 was significantly (P<0.001) diminished by the yarrow oil administration (Fig. 3B).
mucosa with mucin secreting glands (Fig. 4A), while the colon sections of the DSS-induced colitis group showed loss of the epithelial layer and depletion of goblet cells (Fig. 4B); however, treatment with the yarrow oil had protected the mucosa and the mucin glands (Fig. 4C). The level of inflammatory infiltrations was significantly lower in the oil-treated group compared with the DSS-group which showed severe infiltration of many inflammatory cells in the mucosal layer (15.3 ± 1.5 vs. 15.0 ± 3.1, P<0.01) (Fig. 4D). In addition, treatment with the yarrow oil significantly protected the crypt architecture from distraction and interruption of muscularis mucosa in comparison to the DSS-treated group (5.2 ± 1.5 vs. 9.2 ± 3.0, P<0.01) (Fig. 4E).
UC is a chronic inflammatory disease; manifested with a variety of symptoms including diarrhea, tenesmus and abdominal pain and sometimes bloody stool in severe cases. Yarrow has been used traditionally for digestive problems; liver and gallbladder conditions, cramps, fever, and wound healing in addition to its antibacterial and antifungal effects. Many recent researches have been reported on the pharmacological effects of yarrow as anti-inflammatory and immunomodulatory activities [13], and the majority of these medicinal and pharmacological effects are mainly due to the plant essential oil [25].
The study, hereby, showed that the yarrow oil could suppress DSS-induced colitis in mice model and improve colonic injury. Furthermore, treatment with yarrow oil could inhibit pro-inflammatory responses, indicating that the yarrow oil may have a potent anti-inflammatory effect on UC, which was evinced by the normalization of the DAI score. Notably, treatment with yarrow oil reduced losses of crypts, edema, and mucosal erosions in the mucosa determined by the histopathological analysis. In colitis induced by DSS in mice, an increase in colonic infiltration of T-cells, macrophages and neutrophils is usually observed [25]. Yarrow oil was able to inhibit the T-cells, neutrophils, and macrophages infiltrations to ameliorate DSS-induced acute colitis, however, the mechanism of this inhibition needs further investigation.
Yarrow oil showed the ability to suppress UC, so we tried to investigate the potential mechanisms that might explain this therapeutic effect. Although, the exact mechanism of UC is still partly unknown, the NF-κB signaling pathway has been far believed to be able to mediate and maintain remission in UC [24]. NF-κB, a transcription factor, plays a pivotal role in the regulation of inflammation, innate immunity, and tissue integrity. NF-κB is able to mediate pro-inflammatory responses by up-regulating the infiltration of neutrophils and macrophages [21]. Our results show that treatment with the yarrow oil significantly induced a significant increase in the anti-inflammatory cytokine, IL-10, and decreased level of inflammatory cytokine, IL-6, in the same time decreased expression of NF-κB and its signal target gene TNF-α. Most likely, the regulation of NF-κB is a key component of the anti-inflammatory activity of yarrow oil.
Furthermore, it has been reported that the DSS-induced colitis model is characterized by increased serum levels of IL-6, IL-17, and TNF-α and decreased levels of IL-4 and IL-10 [24]. Disturbed production of cytokines is an important finding in UC pathophysiology as these cytokines make a complex interplay that mediates colonic mucosal inflammation and influences the integrity of epithelial layer. The disturbed cytokines include an increase of pro-inflammatory cytokines as IL-1β, IL-6, TNF-α, and interferon-γ (IFN-γ) [27]. In this study, yarrow oil restored significantly the serum levels of both IL-6 and IL-10 in the UC model.
TGF-β regulates mucosal immune reactions via the TGF-β signaling pathway. This pathway is impaired in the colons of IBD patients. Dysregulated signal pathway of TGF-β in the T-cells and dendritic cells causes spontaneous colitis in the mouse model [27]. Study of the inflammatory pathways and immune response of IBD indicated that colonic mucosal damage is controlled by clusters of cells and cytokines [28-30]. A variety of cells including, macrophages, T helper (Th), and regulatory T cells, play a critical role by suppression or enhancement of inflammation. Signaling pathways of cytokines are important in the inflammation process where pro-inflammatory cytokines as TNF-α and IL-6 play an important role in upregulation, while anti-inflammatory cytokines as TGF-β and IL-10 play the opposite role in the downregulation of inflammation [31-35]. In UC there are immunoregulatory abnormalities including the ratio of TNF-α, and IL-6 to IL-10 and TGF-β and selective activation of T cells [28].
There are 2 pathways for T helper 17 (Th17) differentiation have been reported; TGF-β dependent and independent pathway, however, the explanation of the 2 pathways remains controversial [35]. Most of the studies reported that T cell differentiation is TGF-β (family) dependent pathway in concert with IL-6 or IL-21 which differentiate naïve T-cells into Th17 cells, through inducing nuclear receptor retinoic acid receptor-related orphan receptor gamma (ROR-γt) expression [31,33,35,36]. However, some studies reported that Th17 differentiation can be done through TGF-β independent pathway. Jones et al [37]. reported that IL-6-mediates Th17 subset maturation and attributed it to the role of STAT3 also Li et al. [38] proposed that IL-6 promotes T cell proliferation due to low-level expression of the ROR-γt transcription factor under inflammatory conditions only. In our study, yarrow oil treatment boosted TGF-β expression in the colonic tissue during the acute phase of colitis. TGF-β is important for intestinal mucosal immunity via T cell development, homeostasis, and differentiation perhaps with other signaling pathways.
In contrast, PPAR-γ is highly expressed in colonic epithelial cells and to a lesser extent in macrophages and lymphocytes and the protein expression in the colon is closely linked to intestinal-microbial interaction [24]. Using a variety of quantitative methods, expression of PPAR-γ is decreased by at least 60% in the colon of UC patients [39]. This impaired expression is found in both inflamed and non-inflamed parts of the colon and limited only to epithelial cells. This notice suggests that the modified expression is not secondary to the inflammatory process [40]. In contrast, PPAR-γ expression was found to be significantly reduced in the mucosa of active UC patients and it is significantly correlated with disease activity [41]. Recently, PPAR-γ has been reported to play an important role in the immune response via its ability to suppress the expression of a lot of the inflammatory cytokines [42]. In our study, oral administration of yarrow oil reduced the severity of UC symptoms and this was accompanied with enhanced production of the PPAR-γ protein.
The main constituents of yarrow oil are mainly sesquiterpenes such as germacrene D (26.15%), chamazulene (10.04%) and β-caryophyllene (10.35%) and some monoterpenes such as sabinene (14.28%), β-pinene (11.40%) and borneol (5.26%) and this is in accordance with the essential oil composition reported elsewhere [10-12]. The oil is blue in color due to the presence of chamazulene which is known for its anti-inflammatory activity and many studies attributed the anti-inflammatory activity of yarrow and chamomile essential oils to their high concentrations of chamazulene [43]. β-Caryophyllene is also distinguished for its anti-inflammatory and GI tract calming activities [44,45]. The essential oil of Ageratum fastigiatum possessed an anti-inflammatory activity, which was attributed to its high content of germacrene D (24.15%) and α-humulene (11.15%) [45].
The anti-inflammatory effects of most of these components have been reported individually. For instance, both β-caryophyllene and germacrene D reduces the levels of IL-1β, IL-6, TNF-α, and IFN-γ in lipopolysaccharides (LPS)-stimulated cells [46]. Chamazulene attenuates levels of reactive oxygen species in endothelial cells and protects from the vascular damage [47]. 1,8-Cineole ameliorates the GI inflammation and ulceration via reduction of myeloperoxidase activity and repletion of glutathione [48]. Another study indicated that 1,8-cineole could be useful in long term treatment of airway inflammation in bronchial asthma through reduction of TNF-α production [49]. Sabinene inhibits the nitric oxide (NO) production [50]. Borneol reduces the level of inflammatory factors including NO, TNF-α and IL-6 in LPS-induced RAW 264.7 macrophages [51]. Borneol improves the efficacy of edaravone against DSS-induced colitis by promoting M2 macrophages polarization via the JAK2-STAT3 signaling pathway [52]. α-Humulene inhibits the TNF-α and IL-1β generation in carrageenan-injected rats, furthermore, reduces the production of prostaglandin E-2, inducible NO synthase and COX-2 expression [53]. As for our knowledge, we are the first to study the potential effect of yarrow oil to ameliorate the DSS-induced inflammation in mice model.
The anti-inflammatory effects of the yarrow oil constituents that have been reported showed their effect on a variety of signaling pathways or their key element mediators. Moreover, some articles showed multiple mechanisms for the anti-inflammatory effect of the same constituent. Collectively this enhances our finding that the yarrow oil exerts a powerful anti-inflammatory effect in a synergistic and intricate manner through reduction of NF-κB production in the DSS-induced colitis and its target TNF-α gene, and enhancement of the expression of PPAR-γ which suppresses the expression of several inflammatory mediators.
Yarrow oil mitigated UC symptoms both macroscopically and histologically in addition to the associated inflammatory biomarkers. In addition to its ability to regulates immune cell infiltration and inflammatory cytokines secretion via regulation of signals including NF-κB and PPAR-γ pathways. Yarrow oil is emerging as a promising, safe, cheap, and effective drug for the effective therapeutic strategy for the IBD however further pharmaceutical formulations and clinical experiments are needed for validation.
Notes
Funding Source
This work was financially supported by the Deanship of Scientific Research at King Faisal University under Nasher Track (Grant No. 186232).
Author Contribution
Conceptualization: Mohamed ME, Mohafez OM, Elsayed SA. Methodology: Mohamed ME, Mohafez OM, Elsayed SA, Madkor HR, Eldien HMS. Project administration: Mohamed ME. Writing-original draft: Mohafez OM. Writing-review and editing: Mohamed ME, Mohafez OM, Elsayed SA. Approval of final manuscript: all authors.
REFERENCES
1. Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013; 58:519–525.

2. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016; 13:13–27.

3. Gordillo J, Zabana Y, Garcia-Planella E, et al. Prevalence and risk factors for colorectal adenomas in patients with ulcerative colitis. United European Gastroenterol J. 2018; 6:322–330.

4. Kim Y, Wu AG, Jaja-Chimedza A, et al. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS One. 2017; 12:e0184709.

5. Tripathi K, Feuerstein JD. New developments in ulcerative colitis: latest evidence on management, treatment, and maintenance. Drugs Context. 2019; 8:212572.

6. Shah R, Peethambaran B. Anti-inflammatory and anti-microbial properties of Achillea millefolium in acne treatment. In : Chatterjee S, Jungraithmayr W, Bagchi D, editors. Immunity and inflammation in health and disease: emerging roles of nutraceuticals and functional foods in immune support. London: Academic Press;2018. p. 241–248.
7. Baretta IP, Felizardo RA, Bimbato VF, et al. Anxiolytic-like effects of acute and chronic treatment with Achillea millefolium L. extract. J Ethnopharmacol. 2012; 140:46–54.

8. Miller FM, Chow LM. Alkaloids of Achillea millefolium L. I. Isolation and characterization of Achilleine1,2. J Am Chem Soc. 1954; 76:1353–1354.

9. de Souza P, Gasparotto A Jr, Crestani S, et al. Hypotensive mechanism of the extracts and artemetin isolated from Achillea millefolium L. (Asteraceae) in rats. Phytomedicine. 2011; 18:819–825.

10. Verma RS, Joshi N, Padalia RC, et al. Chemical composition and allelopathic, antibacterial, antifungal and in vitro acetylcholinesterase inhibitory activities of yarrow (Achillea millefolium L.) native to India. Ind Crops Prod. 2017; 104:144–155.

11. Marzouki H, Piras A, Porcedda S, Falconieri D, Bagdonaite E. Influence of extraction methods on the composition of essential oils of Achillea millefolium L. from Lithuania. J Biodivers Manage Forestry. 2014; 3:1000133.

12. Nadim MM, Malik AA, Ahmad J, Bakshi SK. The essential oil composition of Achillea millefolium L. cultivated under tropical condition in India. World J Agric Sci. 2011; 7:561–565.
13. Tadić V, Arsić I, Zvezdanović J, Zugić A, Cvetković D, Pavkov S. The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflamatory potential in topical application. J Ethnopharmacol. 2017; 199:138–148.

14. Aydin S, Sevindik E. Achillea millefolium L. subsp. millefolium essential oil’s antifungal effect. Eur J Biol Res. 2018; 8:153–156.
15. Applequist WL, Moerman DE. Yarrow (Achillea millefolium L.): a neglected panacea? A review of ethnobotany, bioactivity, and biomedical research. Econ Bot. 2011; 65:209.

16. Ali SI, Gopalakrishnan B, Venkatesalu V. Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: a review. Phytother Res. 2017; 31:1140–1161.

17. Karakaya S, El SN, Karagozlu N, Sahin S, Sumnu G, Bayramoglu B. Microwave-assisted hydrodistillation of essential oil from rosemary. J Food Sci Technol. 2014; 51:1056–1065.

18. Dong S, Chen M, Dai F, et al. 5-Hydroxytryptamine (5-HT)-exacerbated DSS-induced colitis is associated with elevated NADPH oxidase expression in the colon. J Cell Biochem. 2019; 120:9230–9242.

19. Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014; 104:15.25.1–15.25.14.

20. Wanes D, Jabri MA, Tounsi H, et al. Chemical characterization of bioactive components of Rosa canina extract and its protective effect on dextran sulfate sodium-induced intestinal bowel disease in a mouse model. J Med Food. 2020; 23:1109–1119.

21. Alzahrani AM, Hanieh H, Ibrahim HM, et al. Enhancing miR132 expression by aryl hydrocarbon receptor attenuates tumorigenesis associated with chronic colitis. Int Immunopharmacol. 2017; 52:342–351.

22. Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993; 38:1722–1734.

23. Zhang Z, Wu X, Cao S, et al. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget. 2016; 7:31790–31799.

24. Zuo L, Huang Z, Dong L, et al. Targeting delivery of anti-TNFalpha oligonucleotide into activated colonic macrophages protects against experimental colitis. Gut. 2010; 59:470–479.

25. Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007; 2:541–546.

26. Almeida JR, Souza GR, Silva JC, et al. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. ScientificWorldJournal. 2013; 2013:808460.

27. Ihara S, Hirata Y, Koike K. TGF-β in inflammatory bowel disease: a key regulator of immune cells, epithelium, and the intestinal microbiota. J Gastroenterol. 2017; 52:777–787.

28. Fonseca-Camarillo G, Yamamoto-Furusho JK. Immunoregulatory pathways involved in inflammatory bowel disease. Inflamm Bowel Dis. 2015; 21:2188–2193.

29. Katsanos KH, Papadakis KA. Inflammatory bowel disease: updates on molecular targets for biologics. Gut Liver. 2017; 11:455–463.

30. Tatiya-Aphiradee N, Chatuphonprasert W, Jarukamjorn K. Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol. 2018; 30:1–10.

31. MacFarlane EG, Haupt J, Dietz HC, Shore EM. TGF-β family signaling in connective tissue and skeletal diseases. Cold Spring Harb Perspect Biol. 2017; 9:a022269.

32. Moses HL, Roberts AB, Derynck R. The discovery and early days of TGF-β: a historical perspective. Cold Spring Harb Perspect Biol. 2016; 8:a021865.

33. Al-Rejaie SS, Abuohashish HM, Al-Enazi MM, Al-Assaf AH, Parmar MY, Ahmed MM. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J Gastroenterol. 2013; 19:5633–5644.

35. Sanjabi S, Oh SA, Li MO. Regulation of the immune response by TGF-β: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. 2017; 9:a022236.

36. Geginat J, Paroni M, Kastirr I, Larghi P, Pagani M, Abrignani S. Reverse plasticity: TGF-β and IL-6 induce Th1-to-Th17-cell transdifferentiation in the gut. Eur J Immunol. 2016; 46:2306–2310.

37. Jones LL, Alli R, Li B, Geiger TL. Differential t cell cytokine receptivity and not signal quality distinguishes IL-6 and IL-10 signaling during Th17 differentiation. J Immunol. 2016; 196:2973–2985.

38. Li J, Zhong W, Wang W, et al. Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-κB activation. PLoS One. 2014; 9:e87810.

39. Dubuquoy L, Jansson EA, Deeb S, et al. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology. 2003; 124:1265–1276.

40. Annese V, Rogai F, Settesoldi A, Bagnoli S. PPARγ in inflammatory bowel disease. PPAR Res. 2012; 2012:620839.
41. Yamamoto-Furusho JK, Peñaloza-Coronel A, Sánchez-Muñoz F, Barreto-Zuñiga R, Dominguez-Lopez A. Peroxisome proliferator-activated receptor-gamma (PPAR-γ) expression is downregulated in patients with active ulcerative colitis. Inflamm Bowel Dis. 2011; 17:680–681.

42. Martin H. Role of PPAR-gamma in inflammation: prospects for therapeutic intervention by food components. Mutat Res. 2010; 690:57–63.

43. Ramadan M, Goeters S, Watzer B, et al. Chamazulene carboxylic acid and matricin: a natural profen and its natural prodrug, identified through similarity to synthetic drug substances. J Nat Prod. 2006; 69:1041–1045.

44. Tambe Y, Tsujiuchi H, Honda G, Ikeshiro Y, Tanaka S. Gastric cytoprotection of the non-steroidal anti-inflammatory sesquiterpene, beta-caryophyllene. Planta Med. 1996; 62:469–470.

45. Younis NS, Mohamed ME. β-Caryophyllene as a potential protective agent against myocardial injury: the role of toll-like receptors. Molecules. 2019; 24:1929.

46. Sitarek P, Rijo P, Garcia C, et al. Antibacterial, anti-inflammatory, antioxidant, and antiproliferative properties of essential oils from hairy and normal roots of Leonurus sibiricus L. and their chemical composition. Oxid Med Cell Longev. 2017; 2017:7384061.
47. Querio G, Antoniotti S, Foglietta F, et al. Chamazulene attenuates ROS levels in bovine aortic endothelial cells exposed to high glucose concentrations and hydrogen peroxide. Front Physiol. 2018; 9:246.

48. Santos FA, Silva RM, Campos AR, De Araújo RP, Lima Júnior RC, Rao VS. 1,8-cineole (eucalyptol), a monoterpene oxide attenuates the colonic damage in rats on acute TNBS-colitis. Food Chem Toxicol. 2004; 42:579–584.

49. Juergens UR, Dethlefsen U, Steinkamp G, Gillissen A, Repges R, Vetter H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir Med. 2003; 97:250–256.

50. Valente J, Zuzarte M, Gonçalves MJ, et al. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem Toxicol. 2013; 62:349–354.

51. Zou L, Zhang Y, Li W, et al. Comparison of chemical profiles, anti-inflammatory activity, and UPLC-Q-TOF/MS-based metabolomics in endotoxic fever rats between synthetic borneol and natural borneol. Molecules. 2017; 22:1446.

Fig. 1.
Effect of yarrow oil on disease activity index (DAI) score and the colon length in the dextran sulfate sodium (DSS)-induced colitis. (A) The DAI score, represents the loss of body weight, soft stool or diarrhea in addition to the bleeding. (B) Colons length, representing the mean length of colons for each group±standard deviation. (C) Morphology of colon, representing bleeding, formed or bloody stools, appearance and length. aP<0.01, bP<0.001.
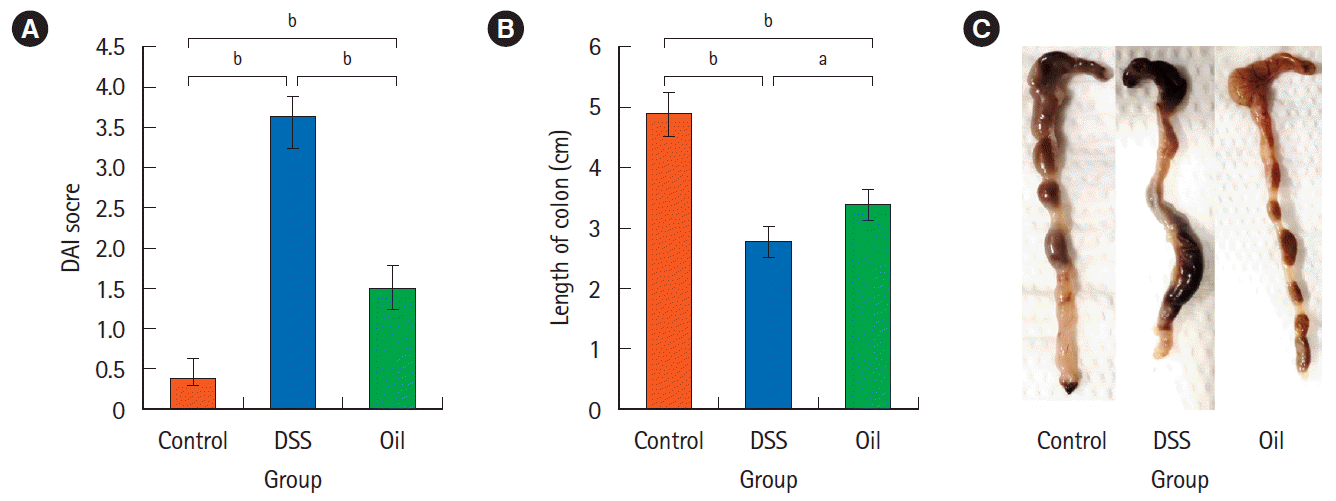
Fig. 2.
Effect of yarrow oil on the expression pattern of the inflammatory cytokines and activation of the NF-κB signal pathway in dextran sulfate sodium (DSS)-induced colitis. Fifty micrograms of colons homogenate was immunoblotted with antibodies specific for NF-κB (A), TNF-α (B), PPAR-γ (C), TGF-β (D) respectively and β-actin was used as a protein loading control. aP<0.05, bP<0.01, cP<0.001. NF-κB, nuclear factor kappa light chain enhancer of activated B cells; TNF-α, tumor necrosis factor-α; PPAR-γ, peroxisome proliferator-activated receptor-γ; TGF-β, transforming growth factor-β; NS, not significant.
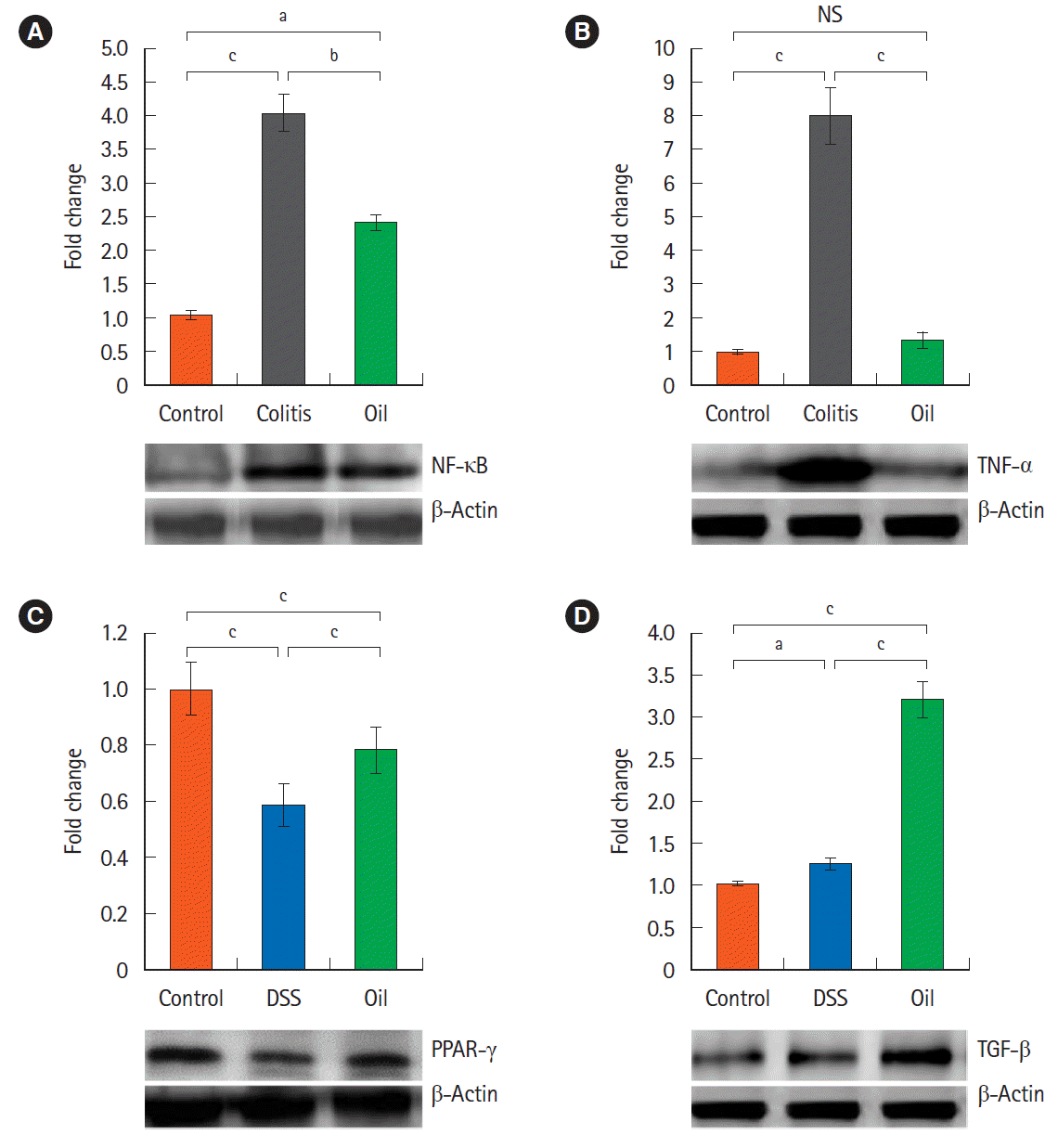
Fig. 3.
Effects of yarrow oil on the serum level of the inflammatory cytokines in dextran sulfate sodium (DSS)-induced colitis. Serum samples were isolated from the collected blood and assayed by ELISA (enzyme-linked immunosorbent assay). (A) The serum level of interleukin 10 (IL-10) and (B) the serum level of IL-6. Data are expressed as mean of 7 mice in each group±standard deviation. aP<0.05, bP<0.01, cP<0.001.

Fig. 4.
The effect of yarrow oil on the histopathological changes in dextran sulfate sodium (DSS)-induced colitis. (A) Control group, (B) DSS-induced colitis, (C) yarrow oil-treated group. The formalin fixed parts of the colon were impeded in paraffin and stained with H&E (×100). Representative sample from each group is shown. (D) Mean score of inflammatory infiltration. (E) Mean score of microscopic crypt destruction. aP<0.01, bP<0.001.

Table 1.
Volatile Constituents from Achillea millefolium Essential Separated and Identified after Gas Chromatography Analysis
| No. | Compound namea | Retention time (min) | RIb | Reported RIc | Area (%)d |
|---|---|---|---|---|---|
| 1 | α-Pinene | 7.678 | 937 | 939 | 1.97 |
| 2 | Camphene | 7.947 | 947 | 946 | 0.28 |
| 3 | Sabinene | 8.632 | 970 | 969 | 14.28 |
| 4 | β-Pinene | 8.948 | 981 | 979 | 11.40 |
| 5 | α-Terpinene | 10.231 | 1019 | 1017 | 0.58 |
| 6 | o-Cymene | 10.554 | 1028 | 1026 | 0.66 |
| 7 | Limonene | 10.603 | 1029 | 1029 | 0.59 |
| 8 | 1,8-Cineole | 10.793 | 1034 | 1031 | 1.87 |
| 9 | Artemisia ketone | 11.946 | 1065 | 1062 | 2.83 |
| 10 | Terpinolene | 12.855 | 1089 | 1088 | 0.49 |
| 11 | Camphor | 15.145 | 1151 | 1146 | 0.49 |
| 12 | Borneol | 15.879 | 1170 | 1165 | 5.26 |
| 13 | Terpinen-4-ol | 16.234 | 1180 | 1177 | 2.11 |
| 14 | Bornyl acetate | 20.284 | 1295 | 1288 | 0.44 |
| 15 | β-Caryophyllene | 24.872 | 1429 | 1419 | 10.35 |
| 16 | α-Humulene | 25.763 | 1457 | 1452 | 1.73 |
| 17 | Germacrene D | 26.859 | 1492 | 1485 | 26.15 |
| 18 | Caryophyllene oxide | 29.991 | 1591 | 1582 | 2.66 |
| 19 | Chamazulene | 34.264 | 1741 | 1731 | 10.04 |
| Total | 94.19 |
c All reported retention indices (RI) are from Almeida et al. [26]
Table 2.
Oil Components Classified According to Common Structures
Table 3.
Histological Score for Quantification the Degree of Colitis




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download