Abstract
Background/Aims
Methods
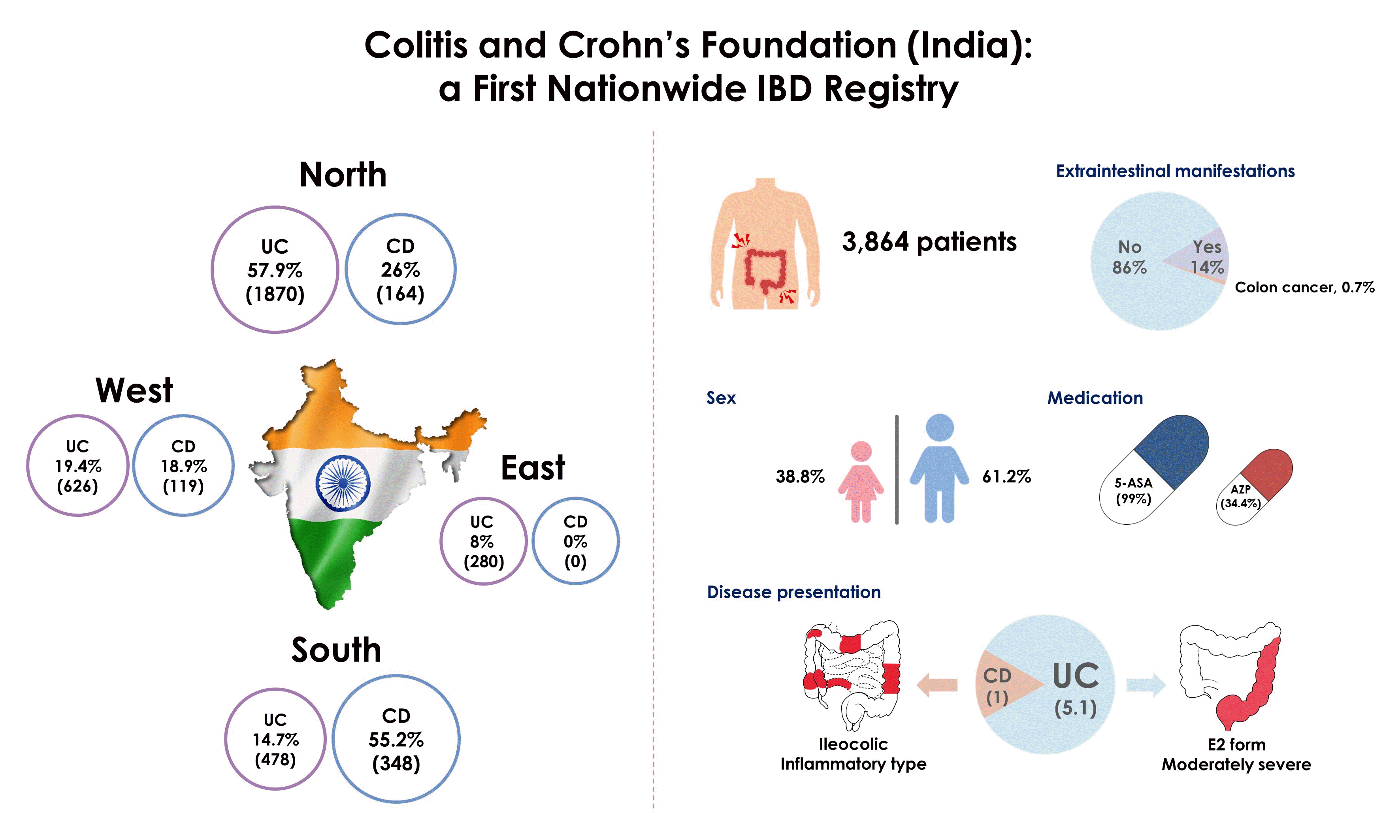
Results
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Sood A and Puri AS are editorial board members of the journal but did not involve in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Conception and design: Sood A, Midha V. Supervision, analysis and interpretation of the data: Sood A, Midha V. Collection: Kaur K, Mahajan R, Singh A, Sharma S, Puri AS, Goswami B, Desai D, Pai CG, Peddi K, Philip M, Kochhar R, Nijhawan S, Bhatia S, Rao NS. Drafting of the article: all authors. Critical revision of the article for important intellectual content: all authors. Final approval of the article: all authors.
REFERENCES
Table 1.
Table 2.
| Clinical presentation | Total |
Ulcerative colitis |
Crohn’s disease |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| North | East | West | South | Total | North | Easta | West | South | Total | |||
| Montreal classification | - | |||||||||||
| E1 | 565 (17.5) | 386 (20.6) | 20 (7.7) | 70 (11.2) | 89 (18.7) | 565 (17.5) | - | - | - | - | ||
| E2 | 1,895 (58.6) | 1,050 (56.1) | 238 (91.5) | 341 (54.5) | 266 (55.9) | 1,895 (58.6) | - | - | - | - | ||
| E3 | 772 (23.9) | 434 (23.2) | 2 (0.8) | 215 (34.3) | 121 (25.4) | 772 (23.9) | - | - | - | - | ||
| A1 | 34 (5.4) | - | - | - | - | - | 9 (5.5) | 2 (1.7) | 23 (6.6) | 34 (5.4) | ||
| A2 | 387 (61.3) | - | - | - | - | - | 82 (50.0) | 96 (80.7) | 209 (60.1) | 387 (61.3) | ||
| A3 | 210 (33.3) | - | - | - | - | - | 73 (44.5) | 21 (17.6) | 116 (33.3) | 210 (33.3) | ||
| B1 | 504 (79.9) | - | - | - | - | - | 125 (76.2) | 72 (60.5) | 307 (88.2) | 504 (79.9) | ||
| B2 | 106 (16.8) | - | - | - | - | - | 33 (20.1) | 47 (39.5) | 26 (7.5) | 106 (16.8) | ||
| B3 | 21 (3.3) | - | - | - | - | - | 6 (3.7) | 0 | 15 (4.3) | 21 (3.3) | ||
| L1 | 194 (30.8) | - | - | - | - | - | 76 (46.3) | 16 (13.4) | 102 (29.3) | 194 (30.8) | ||
| L2 | 195 (30.9) | - | - | - | - | - | 55 (33.5) | 71 (59.7) | 69 (19.8) | 195 (30.9) | ||
| L3 | 229 (36.3) | - | - | - | - | - | 26 (15.9) | 32 (26.9) | 171 (49.1) | 229 (36.3) | ||
| L4 | 13 (2.1) | - | - | - | - | - | 7 (4.3) | 0 | 6 (1.7) | 13 (2.1) | ||
| P | 10 (1.6) | - | - | - | - | - | 2 (1.2) | 0 | 8 (2.3) | 10 (1.6) | ||
| Disease severityb | - | |||||||||||
| Mild | 721 (18.7) | 179 (9.6) | 198 (76.2) | 207 (33.1) | 43 (9.0) | 627 (19.4) | 13 (7.9) | 31 (26.1) | 50 (14.4) | 94 (14.9) | ||
| Moderate | 2,354 (60.9) | 1,394 (74.5) | 60 (23.1) | 253 (40.4) | 232 (48.7) | 1,939 (60.0) | 148 (90.2) | 80 (67.2) | 187 (53.7) | 415 (65.8) | ||
| Severe | 788 (20.4) | 297 (15.9) | 2 (0.8) | 166 (26.5) | 201 (42.2) | 666 (20.6) | 3 (1.8) | 8 (6.7) | 111 (31.9) | 122 (19.3) | ||
| Extraintestinal manifestations | - | |||||||||||
| Total | 552 (14.9) | 214 (11.4) | 0 | 116 (18.5) | 92 (19.3) | 422 (13.1) | 30 (18.3) | 9 (7.6) | 91 (26.1) | 130 (20.6) | ||
| Musculoskeletal | 150 (3.9) | 16 (0.9) | 0 | 89 (14.2) | 23 (4.8) | 128 (4.0) | 4 (2.4) | 0 | 18 (5.2) | 22 (3.5) | ||
| Cardiovascular | 5 (0.1) | 0 | 0 | 0 | 3 (0.6) | 3 (0.1) | 1 (0.6) | 0 | 1 (0.3) | 2 (0.3) | ||
| Genitourinary amyloidosis | 111 (2.9) | 65 (3.5) | 0 | 0 | 2 (0.4) | 67 (2.1) | 9 (5.5) | 0 | 35 (10.1) | 44 (7.0) | ||
| Glomerulonephritis | 6 (0.2) | 3 (1.8) | 3 (0.9) | 6 (0.9) | ||||||||
| Hepatobiliary | 81 (2.1) | 2 (0.1) | 0 | 11 (1.8) | 29 (6.1) | 42 (1.3) | 0 | 0 | 39 (11.2) | 39 (6.2) | ||
| Dermatologic | 60 (1.6) | 4 (0.2) | 0 | 20 (3.2) | 10 (2.1) | 34 (1.1) | 2 (1.2) | 5 (4.2) | 19 (5.5) | 26 (4.1) | ||
| Hematological | 21 (0.5) | 0 | 0 | 0 | 12 (2.5) | 12 (0.4) | 0 | 0 | 9 (2.6) | 9 (1.4) | ||
| Neurological (seizures) | 3 (0.1) | 2 (0.1) | 0 | 0 | 0 | 2 (0.1) | 0 | 0 | 1 (0.3) | 1 (0.2) | ||
| Ophthalmological | 54 (1.4) | 2 (0.1) | 0 | 21 (3.4) | 5 (1.1) | 28 (0.9) | 5 (3.0) | 5 (4.2) | 16 (4.6) | 26 (4.1) | ||
| Pulmonary | 3 (0.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (0.9) | 3 (0.5) | ||
| Pancreatitis | 1 (0.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.3) | 1 (0.2) | ||
| Colon cancer | - | |||||||||||
| Yes | 26 (0.7) | 2 (0.1) | 0 | 17 (2.7) | 3 (0.6) | 22 (0.7) | 1 (0.6) | 0 | 3 (0.9) | 4 (0.6) | ||
Table 3.
Table 4.
| Variable | Total |
Ulcerative colitis |
Crohn’s disease |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| North | East | West | South | Total | North | Easta | West | South | Total | ||
| 5-ASA | 3,825 (99.0) | 1,852 (99.0) | 258 (99.2) | 619 (98.9) | 469 (98.5) | 3,198 (98.9) | 160 (97.6) | - | 119 (100) | 348 (100) | 627 (99.4) |
| Corticosteroids | 1,598 (41.4) | 781 (41.8) | 64 (24.6) | 335 (53.5) | 174 (36.6) | 1,354 (41.9) | 84 (51.2) | - | 63 (52.9) | 97 (27.9) | 244 (38.7) |
| Azathioprine | 1,329 (34.4) | 481 (25.7) | 238 (91.5) | 142 (22.7) | 128 (26.9) | 989 (30.6) | 43 (26.2) | - | 66 (55.5) | 231 (66.4) | 340 (53.9) |
| Biologics | 57 (1.5) | 28 (1.5) | 8 (3.1) | 0 | 5 (1.1) | 41 (1.3) | 2 (1.2) | - | 0 | 14 (4.2) | 16 (2.5) |
| ATT | 33 (0.9) | 1 (0.1) | 0 | 1 (0.2) | 2 (0.4) | 3 (0.1) | 1 (0.6) | - | 29 (24.4) | 0 | 30 (4.8) |
| Surgery | 110 (2.8) | 39 (2.1) | 1 (0.4) | 1 (0.2) | 5 (1.1) | 46 (1.4) | 22 (13.4) | - | 28 (23.5) | 14 (4.0) | 64 (10.1) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download