Abstract
Background/Aims
Methods
Results
Notes
Conflict of Interest
Clinical trials were sponsored and conducted by Takeda Pharmaceuticals Company Ltd. Medical writing support was sponsored by Takeda Pharmaceuticals Company Ltd. Ooi CJ has received speaker and travel grants from Takeda, Johnson and Johnson, Celltrion, Abbvie, Shire, and Ferring Pharmaceuticals; and participated in Advisory Boards for Takeda, Johnson and Johnson, Shire, and Ferring Pharmaceuticals. Dirk Demuth, Dirk Lindner, and Shashi Adsul are employees of Takeda Pharmaceuticals Company Ltd. The other authors report no conflict of interest.
Ooi CJ is an editorial board member of the journal but did not involve in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Conceptualization: Ooi CJ, Hilmi IN, Kim HJ, Jalihal U, Wu DC, Demuth D, Lindner D, Adsul S. Methodology: Ooi CJ, Hilmi IN, Kim HJ, Jalihal U, Wu DC, Demuth D, Lindner D, Adsul S. Formal analysis: Demuth D, Lindner D, Adsul S. Project administration: Ooi CJ, Demuth D, Lindner D, Adsul S. Visualization: Ooi CJ, Hilmi IN, Kim HJ, Jalihal U, Wu DC, Demuth D, Lindner D, Adsul S. Writing - original draft: Demuth D, Lindner D, Adsul S. Writing - review and editing: Ooi CJ, Hilmi IN, Kim HJ, Jalihal U, Wu DC, Demuth D, Lindner D, Adsul S. Approval of final manuscript: all authors.
Others
The authors thank the patients and their caregivers in addition to the investigators and their teams who contributed to this study. Medical Writing support was provided by Assansa, India (a Healthcare Consultancy-Assansa consultants Dr. Aamir Shaikh MD, Dr. Saifuddin Kharawala MBBS, DPM) and sponsored by Takeda Pharmaceuticals Company Ltd.
SUPPLEMENTARY MATERIALS
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
REFERENCES
Fig. 1.
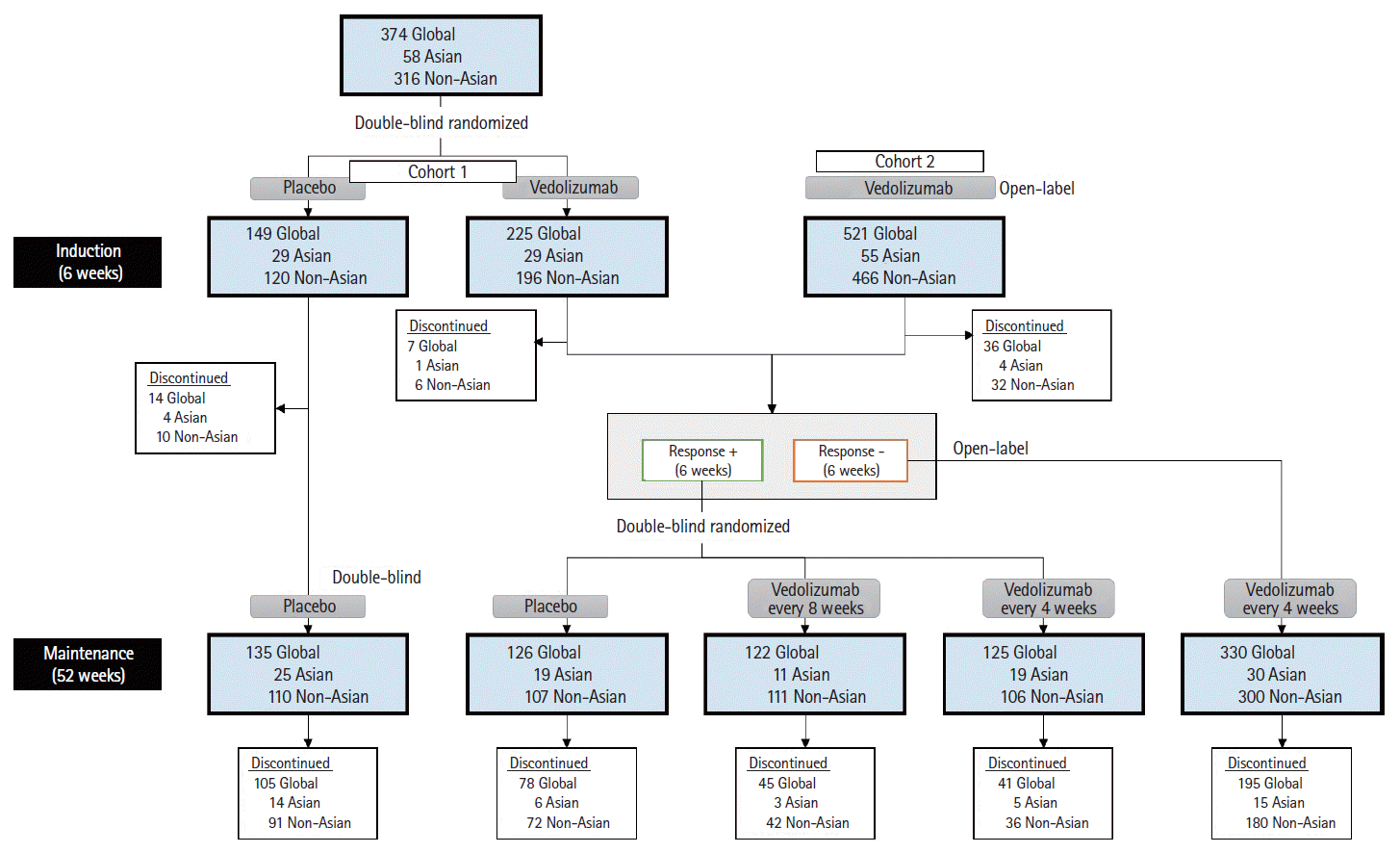
Fig. 2.
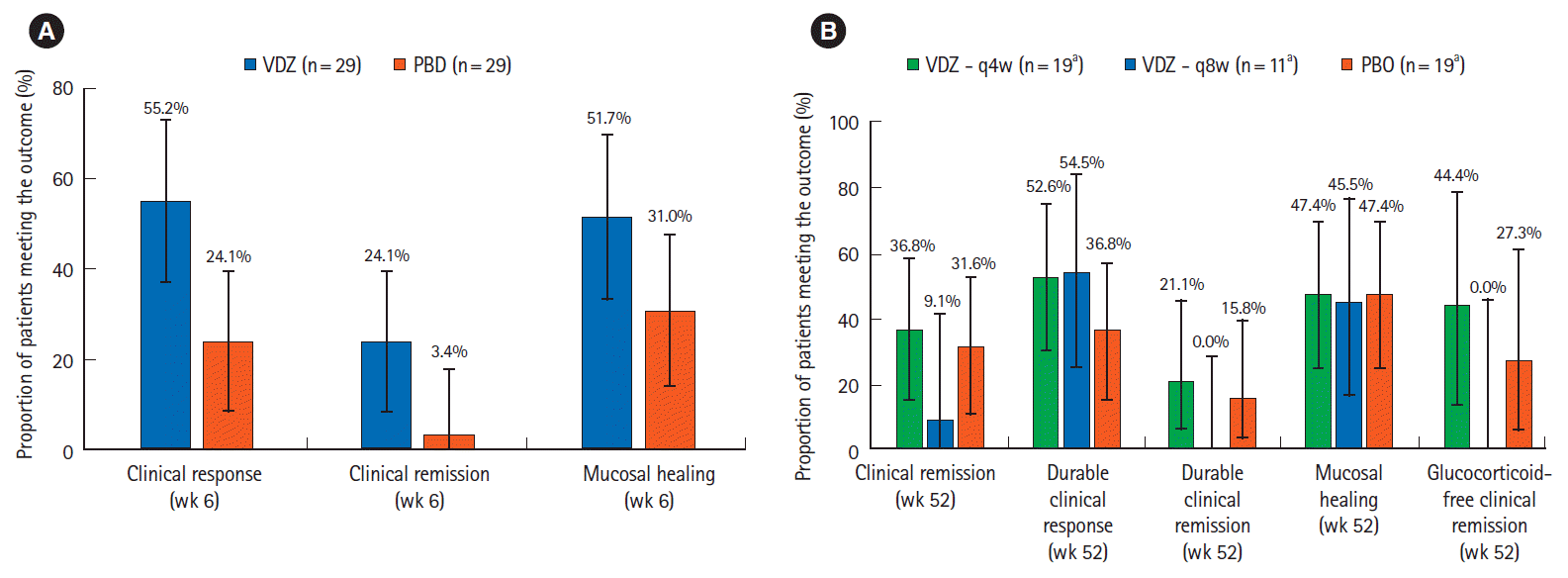
Fig. 3.
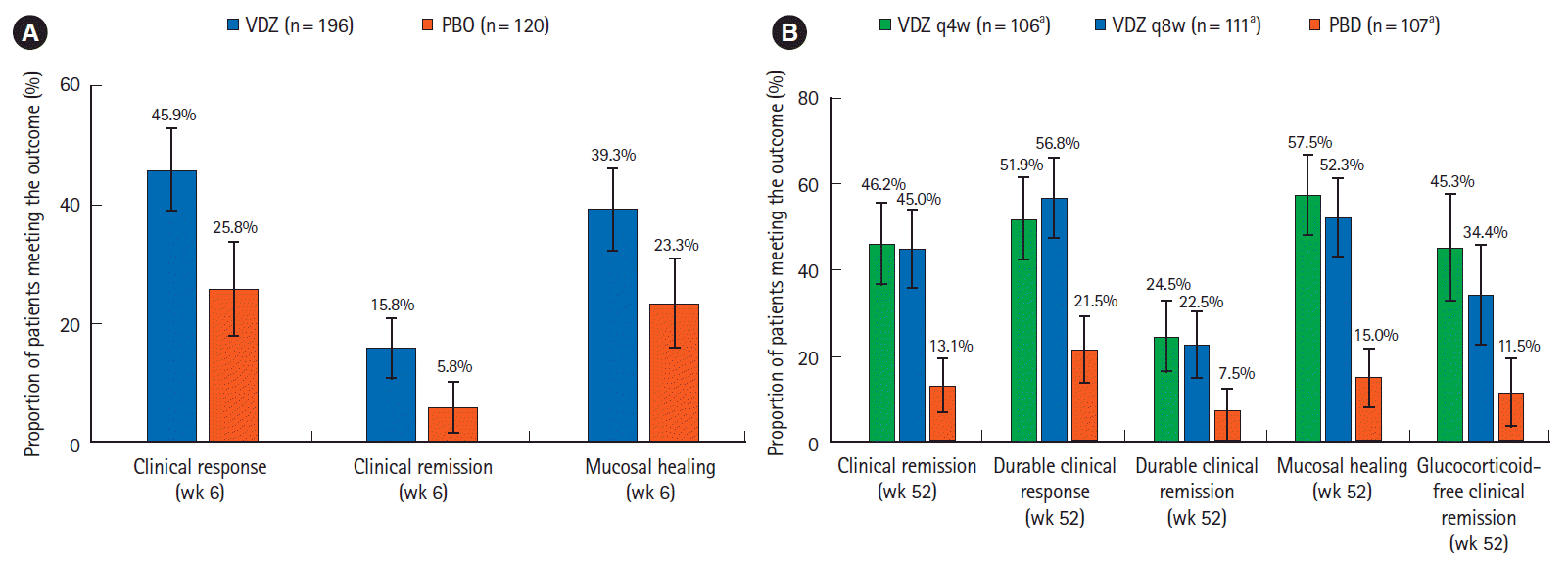
Fig. 4.
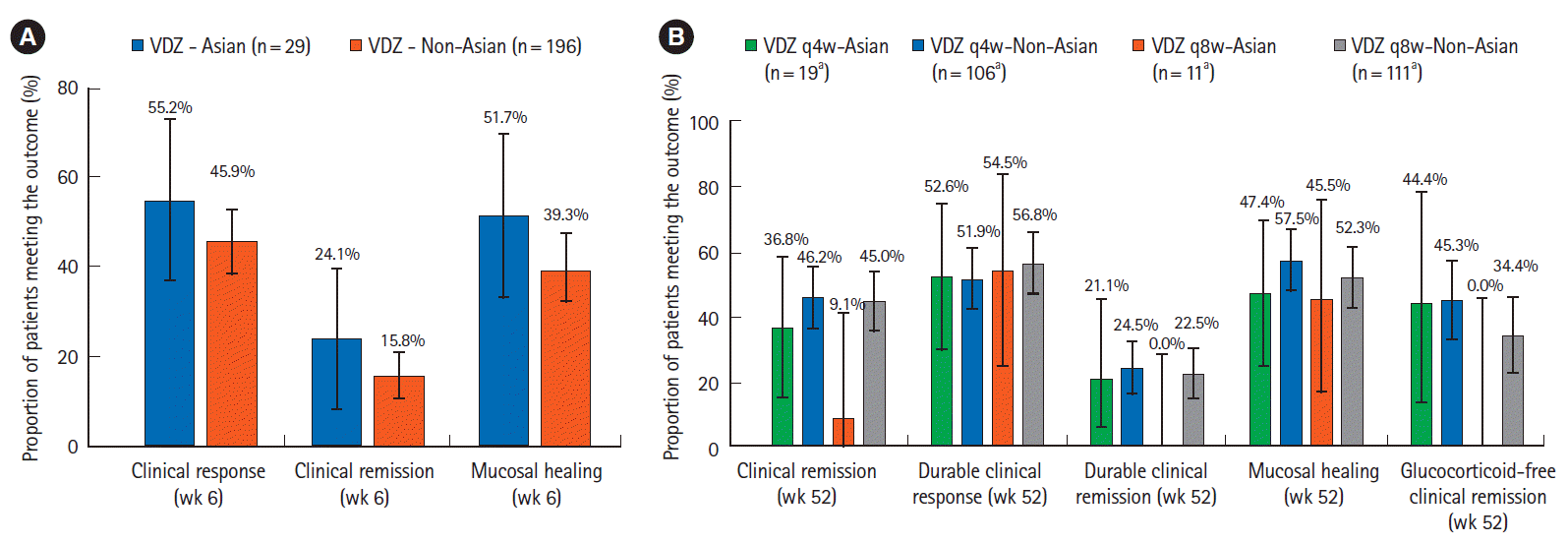
Table 1.
| Parameter | Placeboa | Vedolizumab (cohort 1)a | Vedolizumab (cohort 2) | Vedolizumab (combined) |
|---|---|---|---|---|
| No. | 29 | 29 | 55 | 84 |
| Male sex | 13 (45) | 17 (59) | 29 (53) | 46 (55) |
| Age (yr) | 38.5 ± 11.3 | 36.0 ± 11.3 | 40.7 ± 12.8 | 39.1 ± 12.5 |
| Body weight (kg) | 55.1 ± 12.7 | 57.4 ± 11.1 | 57.0 ± 11.7 | 57.2 ± 11.4 |
| Duration of UC (yr) | 2.5 (0.7–16.6) | 2.6 (0.5–10.0) | 4.2 (0.5–14.1) | 3.5 (0.5–14.1) |
| Concomitant medications for UC | ||||
| Only glucocorticoids | 9 (31) | 6 (21) | 20 (36) | 26 (31) |
| Only immunomodulators | 6 (21) | 8 (28) | 19 (35) | 27 (32) |
| Glucocorticoids and immunomodulators | 8 (28) | 7 (24) | 7 (13) | 14 (17) |
| No glucocorticoids or immunomodulators | 6 (21) | 8 (28) | 9 (16) | 17 (20) |
| Patients with prior anti-TNF use | 2 (7) | 0 | 6 (11) | 6 (7) |
| Patients with prior anti-TNF failure | 2 (7) | 0 | 3 (5) | 3 (4) |
| Complete Mayo score | 8.3 ± 1.3 | 8.7 ± 1.6 | 8.6 ± 1.6 | 8.6 ± 1.6 |
Values are presented as number (%), mean±standard deviation, or median (range).
Placebo and vedolizumab (cohort 1)=the groups that were part of the double-blind induction phase (induction intent-to-treat [ITT] population); Vedolizumab (cohort 2)=additional patients were enrolled to meet the maintenance phase sample size requirements and received open-label vedolizumab (induction safety population only); Vedolizumab combined=all patients that received vedolizumab during the induction phase.
Table 2.
| Parameter |
ITTa |
Non-ITTb |
Placebo combined | Vedolizumab combined | |||
|---|---|---|---|---|---|---|---|
| Placebo | Vedolizumab q8w | Vedolizumab q4w | Placebo | Vedolizumab q4w | |||
| No. | 19 | 11 | 19 | 29 | 35 | 48 | 65 |
| Male sex | 10 (53) | 5 (45) | 9 (47) | 13 (45) | 22 (63) | 23 (48) | 36 (55) |
| Age (yr) | 35.9 ± 13.2 | 46.8 ± 13.1 | 37.9 ± 11.6 | 38.5 ± 11.3 | 39.1 ± 11.8 | 37.5 ± 12.0 | 40.1 ± 12.2 |
| Body weight (kg) | 57.8 ± 11.4 | 62.8 ± 13.5 | 56.6 ± 8.1 | 55.1 ± 12.7 | 55.4 ± 12.1 | 56.2 ± 12.1 | 57.0 ± 11.5 |
| Duration of UC (yr) | 3.9 (1.6–11.1) | 6.5 (0.8–10.2) | 2.7 (0.5–14.1) | 2.5 (0.7–16.6) | 2.8 (0.6–8.7) | 3.0 (0.7–16.6) | 3.1 (0.5–14.1) |
| Concomitant medications for UC | |||||||
| Only glucocorticoids | 6 (32) | 3 (27) | 6 (32) | 9 (31) | 11 (31) | 15 (31) | 20 (31) |
| Only immunomodulators | 7 (37) | 2 (18) | 5 (26) | 6 (21) | 13 (37) | 13 (27) | 20 (31) |
| Glucocorticoids and immunomodulators | 5 (26) | 3 (27) | 3 (16) | 8 (28) | 3 (9) | 13 (27) | 9 (14) |
| No glucocorticoids or immunomodulators | 1 (5) | 3 (27) | 5 (26) | 6 (21) | 8 (23) | 7 (15) | 16 (25) |
| Patients with prior anti-TNF use | 2 (11) | 0 | 1 (5) | 2 (7) | 3 (9) | 4 (8) | 4 (6) |
| Patients with prior anti-TNF failure | 2 (11) | 0 | 0 | 2 (7) | 1 (3) | 4 (8) | 1 (2) |
| Complete Mayo score | 8.3 ± 1.8 | 8.1 ± 1.1 | 8.5 ± 1.5 | 8.3 ± 1.3 | 9.1 ± 1.5 | 8.3 ± 1.5 | 8.8 ± 1.5 |
Values are presented as number (%), mean±standard deviation, or median (range).
Intent-to-treat (ITT)=patients who showed response to vedolizumab at 6 weeks and were randomized as part of the double-blind maintenance phase (maintenance ITT population); Non-ITT placebo=patients that were randomized to placebo during the induction phase and continued to received double-blind placebo during maintenance phase (maintenance safety population only); Non-ITT vedolizumab q4w=patients that did not show response to vedolizumab at 6 weeks and received open-label vedolizumab during the maintenance phase (maintenance safety population only); Placebo combined=all patients that received placebo during the maintenance phase; Vedolizumab combined=all patients that received vedolizumab during the maintenance phase.
b Patient numbers do not exactly match those shown in disposition (Fig. 1) because those patients who were discontinued from the study during the induction phase continued to be included in the safety population and have been counted within these groups.
Table 3.
| Parameter | Placeboa | Vedolizumab (cohort 1)a | Vedolizumab (cohort 2) | Vedolizumab (combined) |
|---|---|---|---|---|
| No. | 120 | 196 | 466 | 662 |
| Male sex | 79 (66) | 115 (59) | 272 (58) | 387 (58) |
| Age (yr) | 41.8 ± 12.7 | 40.7 ± 13.3 | 40.1 ± 13.3 | 40.2 ± 13.3 |
| Body weight (kg) | 76.6 ± 16.1 | 74.6 ± 16.7 | 76.2 ± 19.0 | 75.7 ± 18.4 |
| Duration of UC (yr) | 5.2 (0.5–38.5) | 5.0 (0.5–25.8) | 5.4 (0.5–37.5) | 5.2 (0.5–37.5) |
| Concomitant medications for UC | ||||
| Only glucocorticoids | 49 (41) | 73 (37) | 175 (38) | 248 (37) |
| Only immunomodulators | 12 (10) | 20 (10) | 94 (20) | 114 (17) |
| Glucocorticoids and immunomodulators | 18 (15) | 40 (20) | 69 (15) | 109 (16) |
| No glucocorticoids or immunomodulators | 41 (34) | 63 (32) | 128 (27) | 191 (29) |
| Patients with prior anti-TNF use | 71 (59) | 95 (48) | 257 (55) | 352 (53) |
| Patients with prior anti-TNF failure | 61 (51) | 82 (42) | 219 (47) | 352 (53) |
| Complete Mayo score | 8.7 ± 1.8 | 8.5 ± 1.8 | 8.6 ± 1.8 | 8.5 ± 1.8 |
Values are presented as number (%), mean±standard deviation, or median (range).
Placebo and vedolizumab (cohort 1)=the groups that were part of the double-blind induction phase (induction intent-to-treat [ITT] population); Vedolizumab (cohort 2)=additional patients were enrolled to meet the maintenance phase sample size requirements and received open-label vedolizumab (induction safety population only); Vedolizumab combined=all patients that received vedolizumab during the induction phase.
Table 4.
| Parameter |
ITTa |
Non-ITTb |
Placebo combined | Vedolizumab combined | |||
|---|---|---|---|---|---|---|---|
| Placebo | Vedolizumab q8w | Vedolizumab q4w | Placebo | Vedolizumab q4w | |||
| No. | 107 | 111 | 106 | 120 | 338 | 227 | 555 |
| Male sex | 59 (55) | 65 (59) | 59 (56) | 79 (66) | 204 (60) | 138 (61) | 138 (61) |
| Age (yr) | 41.1 ± 14.0 | 40.5 ± 12.8 | 38.7 ± 14.7 | 41.8 ± 12.7 | 40.4 ± 12.8 | 41.5 ± 13.3 | 40.1 ± 13.2 |
| Body weight (kg) | 77.8 ± 20.2 | 79.7 ± 18.6 | 74.5 ± 16.4 | 76.6 ± 16.1 | 74.2 ± 18.1 | 77.2 ± 18.1 | 75.4 ± 18.0 |
| Duration of UC (yr) | 5.8 (0.5–29.7) | 5.4 (0.7–26.3) | 5.6 (0.7–26.3) | 5.2 (0.5–38.5) | 5.0 (0.5–35.4) | 5.5 (0.5–38.5) | 5.0 (0.5–37.5) |
| Concomitant medications for UC | |||||||
| Only glucocorticoids | 42 (39) | 45 (41) | 42 (40) | 49 (41) | 119 (35) | 91 (40) | 206 (37) |
| Only immunomodulators | 20 (19) | 19 (17) | 15 (14) | 12 (10) | 60 (18) | 32 (14) | 94 (17) |
| Glucocorticoids and immunomodulators | 5 (26) | 3 (27) | 3 (16) | 18 (15) | 49 (14) | 37 (16) | 90 (16) |
| No glucocorticoids or immunomodulators | 26 (24) | 28 (25) | 27 (25) | 41 (34) | 110 (33) | 67 (30) | 165 (30) |
| Patients with prior anti-TNF use | 45 (42) | 50 (45) | 51 (48) | 45 (42) | 50 (45) | 71 (59) | 206 (61) |
| Patients with prior anti-TNF failure | 36 (34) | 43 (39) | 40 (38) | 36 (34) | 43 (39) | 49 (41) | 49 (41) |
| Complete Mayo score | 8.4 ± 1.8 | 8.5 ± 1.9 | 8.3 ± 1.7 | 8.4 ± 1.8 | 8.5 ± 1.9 | 8.7 ± 1.8 | 8.7 ± 1.8 |
Values are presented as number (%), mean±standard deviation, or median (range).
Intent-to-treat (ITT)=patients who showed response to vedolizumab at 6 weeks and were randomized as part of the double-blind maintenance phase (maintenance ITT population); Non-ITT placebo=patients that were randomized to placebo during the induction phase and continued to received double-blind placebo during maintenance phase (maintenance safety population only); Non-ITT vedolizumab q4w=patients that did not show response to vedolizumab at 6 weeks and received open-label vedolizumab during the maintenance phase (maintenance safety population only); Placebo combined=all patients that received placebo during the maintenance phase; Vedolizumab combined=all patients that received vedolizumab during the maintenance phase.
b Patient numbers do not exactly match those shown in disposition (Fig. 1) because those patients who were discontinued from the study during the induction phase continued to be included in the safety population and have been counted within these groups.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download