Abstract
Background/Aims
Methods
Results
Notes
Funding Source
This study was supported by the Deutsche Forschungsgemeinschaft, Germany (Heisenberg Program).
Author Contribution
Study concept and design: Keller R, Malek NP, Wehkamp J, Enck P, Klag T. Acquisition of data: Keller R, Fantasia L, Enck P, Klag T. Analysis and interpretation of data: Keller R, Mazurak N, Fantasia L, Fusco S, Enck P, Klag T. Drafting of the manuscript: Keller R, Enck P, Klag T. Critical revision of the manuscript for important intellectual content: Mazurak N, Fantasia L, Fusco S, Malek NP, Wehkamp J, Enck P, Klag T. Statistical analysis: Keller R, Mazurak N, Fantasia L, Enck P, Klag T. Obtained funding: Wehkamp J, Klag T. Technical, or material support: Wehkamp J, Enck P, Klag T. Study supervision: Malek NP, Wehkamp J, Enck P, Klag T. Approval of final manuscript: all authors.
Supplementary Materials
Supplementary Table 1.
REFERENCES
Fig. 1.
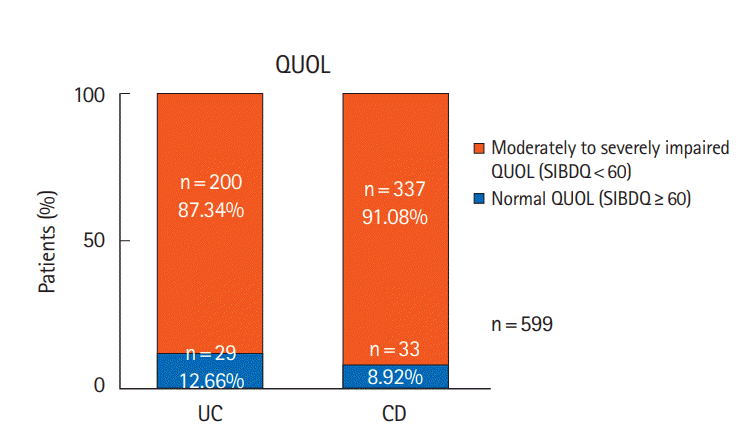
Fig. 2.
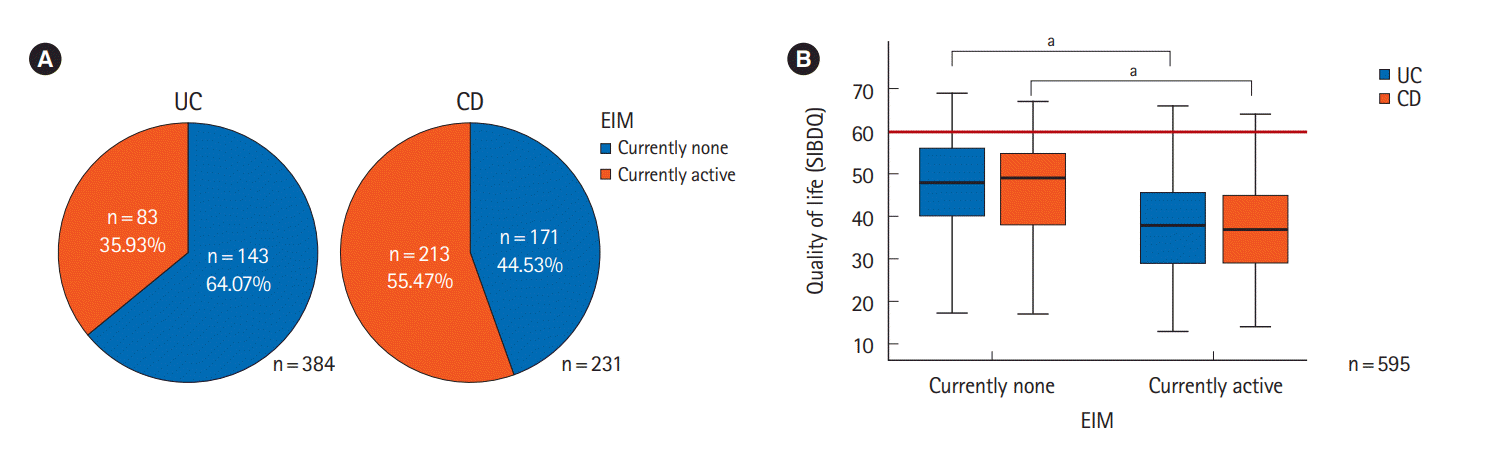
Fig. 3.
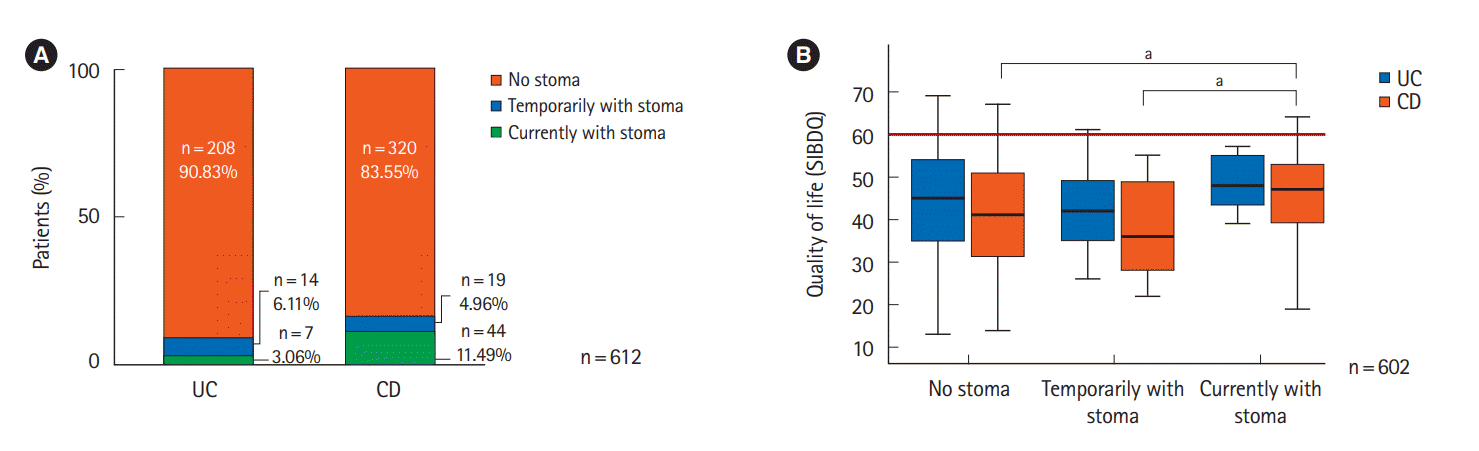
Fig. 4.
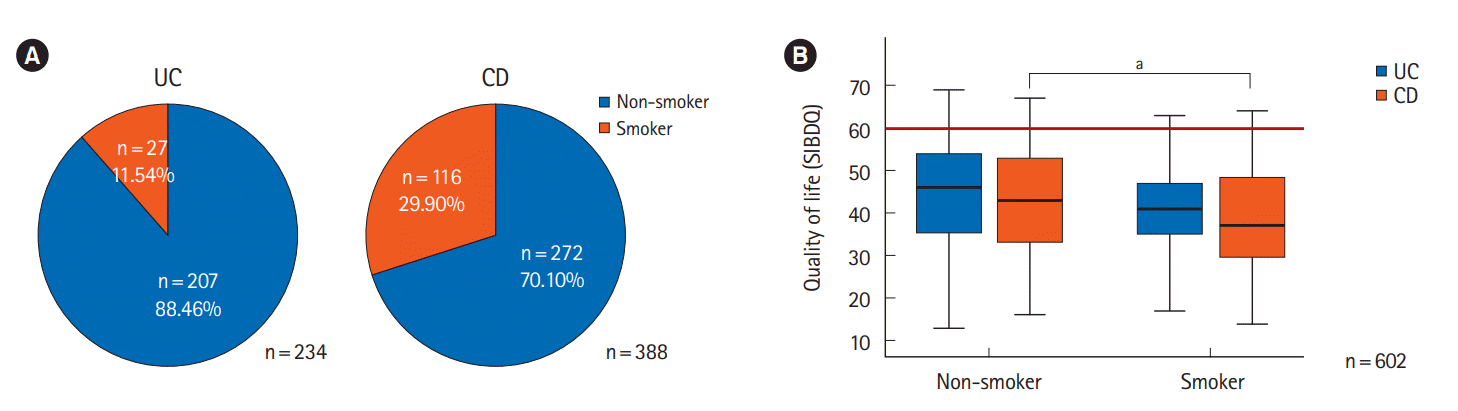
Fig. 5.
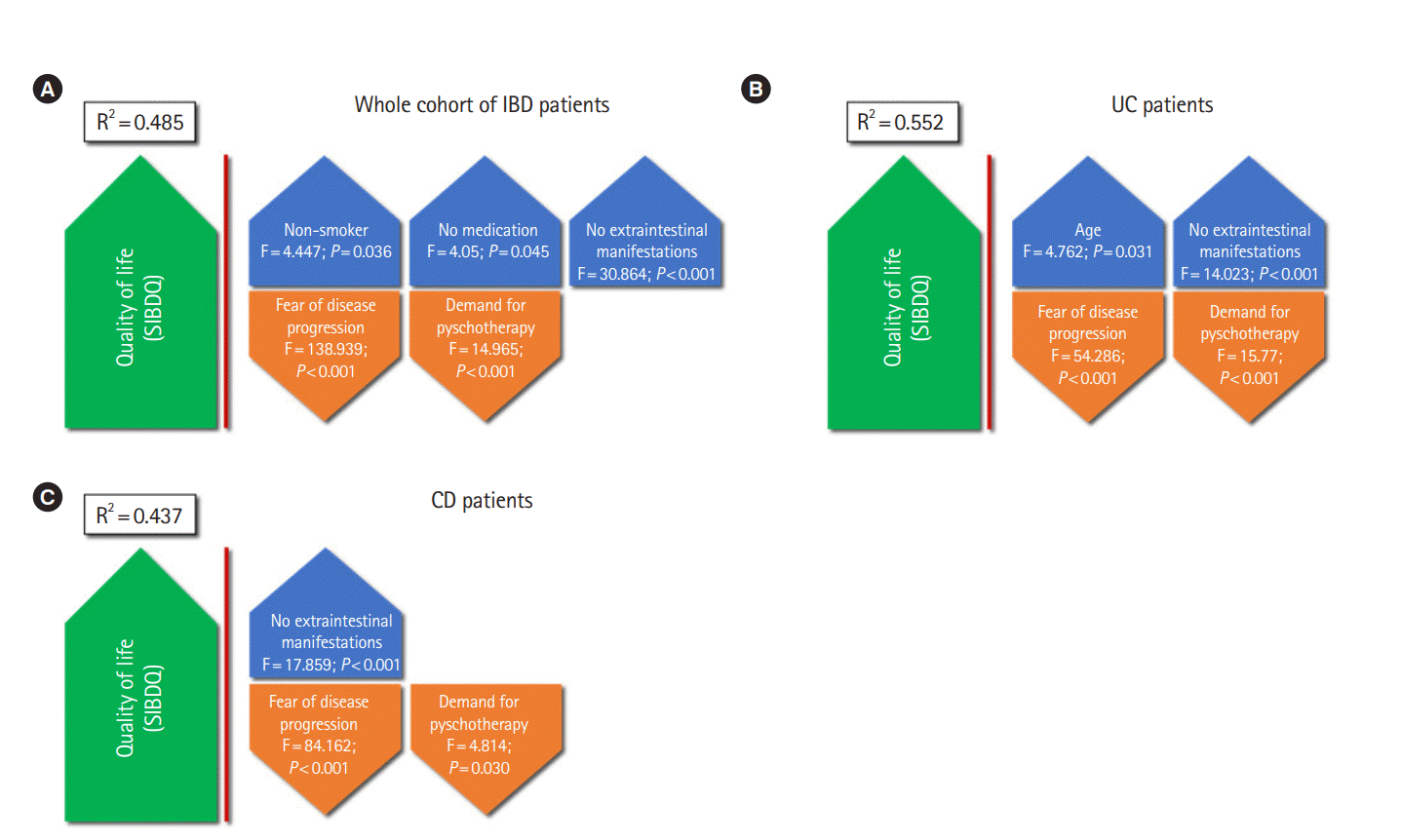
Table 1.
| Characteristic |
Quality of life (SIBDQ score) |
|||||
|---|---|---|---|---|---|---|
|
IBD |
UC |
CD |
||||
| Mean ± SD | P-valuea | Mean ± SD | P-valuea | Mean ± SD | P-valuea | |
| Sex | 0.009 | 0.668 | 0.003 | |||
| Male | 45.0 ± 12.3 | 44.8 ± 12.8 | 45.6 ± 11.8 | |||
| Female | 42.0 ± 12.2 | 44.0 ± 12.4 | 40.9 ± 12.1 | |||
| Age (yr) | 0.009 | 0.055 | 0.040 | |||
| ≤ 30 | 40.1 ± 12.5 | 42.4 ± 13.3 | 39.9 ± 11.9 | |||
| > 30 | 43.7 ± 12.2 | 45.7 ± 11.9 | 42.7 ± 12.3 | |||
| Duration of the IBD (yr) | 0.001b | < 0.001b | 0.317b | |||
| 0–5 | 39.9 ± 12.3 | 40.0 ± 12.7 | 40.0 ± 12.1 | |||
| 6–10 | 41.5 ± 11.4 | 41.9 ± 10.8 | 41.1 ± 12.0 | |||
| 11–15 | 44.9 ± 12.1 | 48.8 ± 11.8 | 42.7 ± 11.7 | |||
| > 15 | 44.5 ± 12.6 | 48.6 ± 12.1 | 42.9 ± 12.4 | |||
| No. of prescribed pharmaceuticals for IBD | < 0.001 | < 0.001 | < 0.001 | |||
| None | 48.9 ± 12.1 | 53.9 ± 12.6 | 47.4 ± 11.9 | |||
| 1 | 45.0 ± 12.2 | 45.4 ± 12.1 | 44.8 ± 12.3 | |||
| 2–3 | 42.2 ± 11.7 | 44.9 ± 11.5 | 40.4 ± 11.5 | |||
| ≥4 | 36.3 ± 11.9 | 36.8 ± 13.5 | 36.1 ± 11.0 | |||
| Undergone IBD surgery | 0.665 | 0.911 | 0.081 | |||
| No | 42.5 ± 12.8 | 44.1 ± 12.8 | 40.4 ± 12.5 | |||
| Yes | 42.9 ± 11.8 | 44.4 ± 11.3 | 42.7 ± 11.9 | |||
| Psychotherapy experience | 0.038 | 0.060 | 0.317 | |||
| No | 43.7 ± 12.3 | 45.7 ± 12.3 | 42.4 ± 12.2 | |||
| Yes | 41.6 ± 12.4 | 42.5 ± 12.7 | 41.6 ± 12.2 | |||
| Extraintestinal manifestations | < 0.001 | < 0.001 | < 0.001 | |||
| No | 47.1 ± 11.4 | 47.3 ± 11.6 | 47.0 ± 11.3 | |||
| Yes | 38.9 ± 11.5 | 38.4 ± 12.1 | 37.7 ± 11.3 | |||
| Smoking | < 0.001 | 0.001 | ||||
| No | 43.7 ± 12.4 | 44.5 ± 12.7 | 43.1 ± 12.1 | |||
| Yes | 39.0 ± 11.4 | 41.2 ± 10.1 | 38.5 ± 11.6 | |||
| Stoma | 0.029 | 0.604 | 0.014 | |||
| No | 42.4 ± 12.5 | 44.1 ± 12.8 | 41.4 ± 12.3 | |||
| Yes, transient | 39.6 ± 11.5 | 42.9 ± 11.6 | 37.2 ± 11.1 | |||
| Yes, currently | 46.5 ± 10.7 | 48.6 ± 7.2 | 46.2 ± 11.3 | |||
| DCCV member | < 0.001 | < 0.001 | 0.004 | |||
| No | 40.0 ± 12.6 | 40.6 ± 13.4 | 39.6 ± 12.2 | |||
| Yes | 44.9 ± 11.9 | 47.3 ± 11.3 | 43.5 ± 12.2 | |||
| Fear of progression (FoP-Q-SF) | < 0.001 | < 0.001 | < 0.001 | |||
| ≥ 36 | 51.1 ± 10.2 | 51.8 ± 10.0 | 50.5 ± 10.4 | |||
| < 36 | 37.1 ± 10.3 | 37.2 ± 10.3 | 37.0 ± 10.4 | |||
| Demand for psychotherapy (ADAPT) | < 0.001 | < 0.001 | < 0.001 | |||
| < 60 | 46.9 ± 11.7 | 50.0 ± 10.7 | 45.1 ± 11.9 | |||
| ≥ 60 | 39.3 ± 11.7 | 39.0 ± 11.6 | 39.5 ± 11.8 | |||
SIBDQ, Short Inflammatory Bowel Disease Questionnaire; IBD, inflammatory bowel disease; UC, ulcerative colitis; CD, Crohn’s disease; SD, standard deviation; DCCV, Deutsche Morbus Crohn/Colitis ulcerosa Vereinigung; Fop-Q-SH, Fear of Progression Questionnaire Short Form; ADAPT, Assessment of the Demand for Additional Psychological Treatment.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download