Abstract
Background/Aims
Methods
Results
Conclusions
Notes
Conflict of Interest
Hibi T has received grants from AbbVie, EA Pharmaceutical, JIMRO, Otsuka Holdings, and Zeria Pharmaceutical; lecture fees from Aspen Japan KK, AbbVie GK, Ferring, Gilead Sciences, Janssen, JIMRO, Kisse Pharmaceutical, Mitsubishi-Tanabe Pharmaceutical, Mochida Pharmaceutical, Nippon Kayaku, Pfizer, Takeda Pharmaceutical, and Zeria Pharmaceutical. Kamae I has received research grants from Takeda, Crecon Medical Assessment, Ono Pharmaceutical Co, Ltd, and Becton, Dickinson and Company Japan; traveling expense compensated by Takeda to attend the Takeda global advisory meeting (to Istanbul, once). Iwakiri R and Ursos L are employed by Takeda. Patel H was an employee of Takeda at the time the research was performed. Hather G and Pinton P are employed by and own stocks in Takeda. But, no other potential conflict of interest relevant to this article was reported.
Hibi T is an editorial board member of the journal but did not involve in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author Contribution
Study concept and design: Pinton P, Ursos L, Patel H. Literature review: Pinton P, Ursos L. Interpretation of data and adding clinical context: Hibi T, Kamae I, Pinton P, Ursos L, Iwakiri R, Hather G, Patel H. Drafting of the manuscript: Hibi T, Kamae I, Pinton P, Ursos L, Iwakiri R, Hather G, Patel H. All authors critically reviewed and revised the manuscript for important intellectual content and approved the final version of the manuscript before submission.
SUPPLEMENTARY MATERIALS
REFERENCES
Fig. 1.
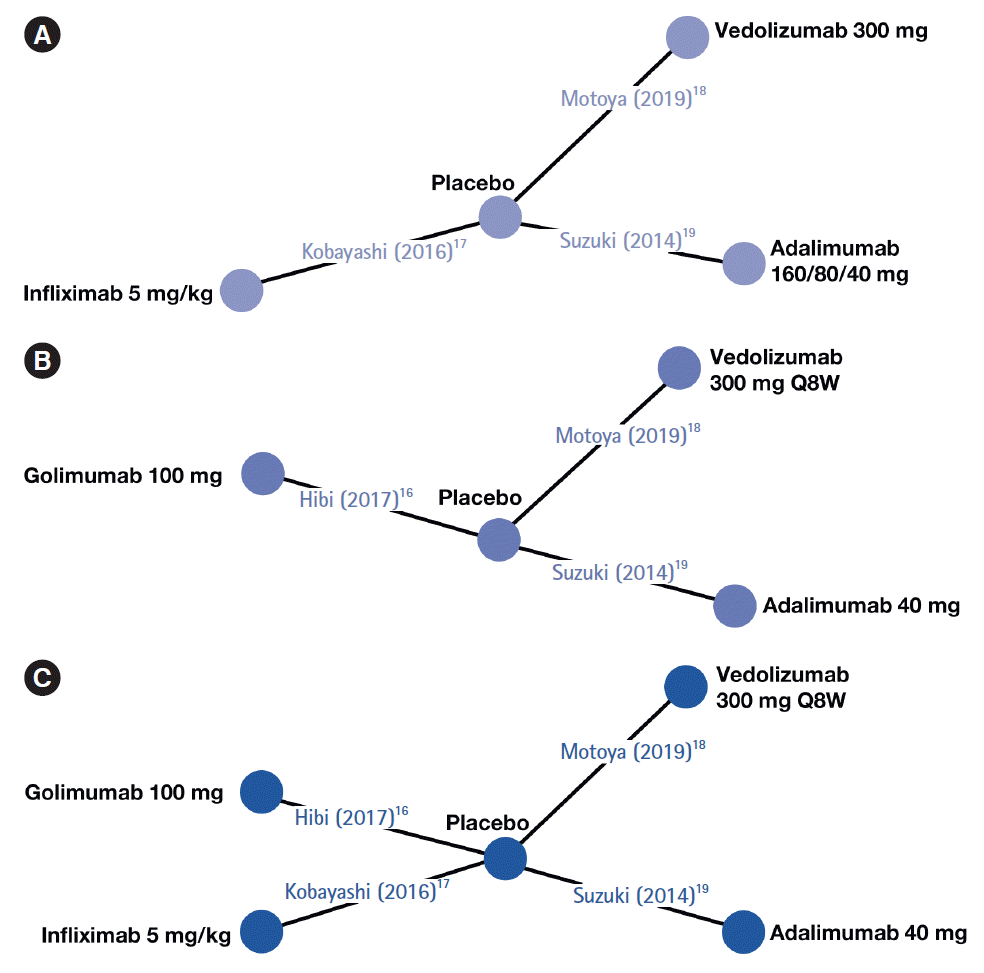
Fig. 2.
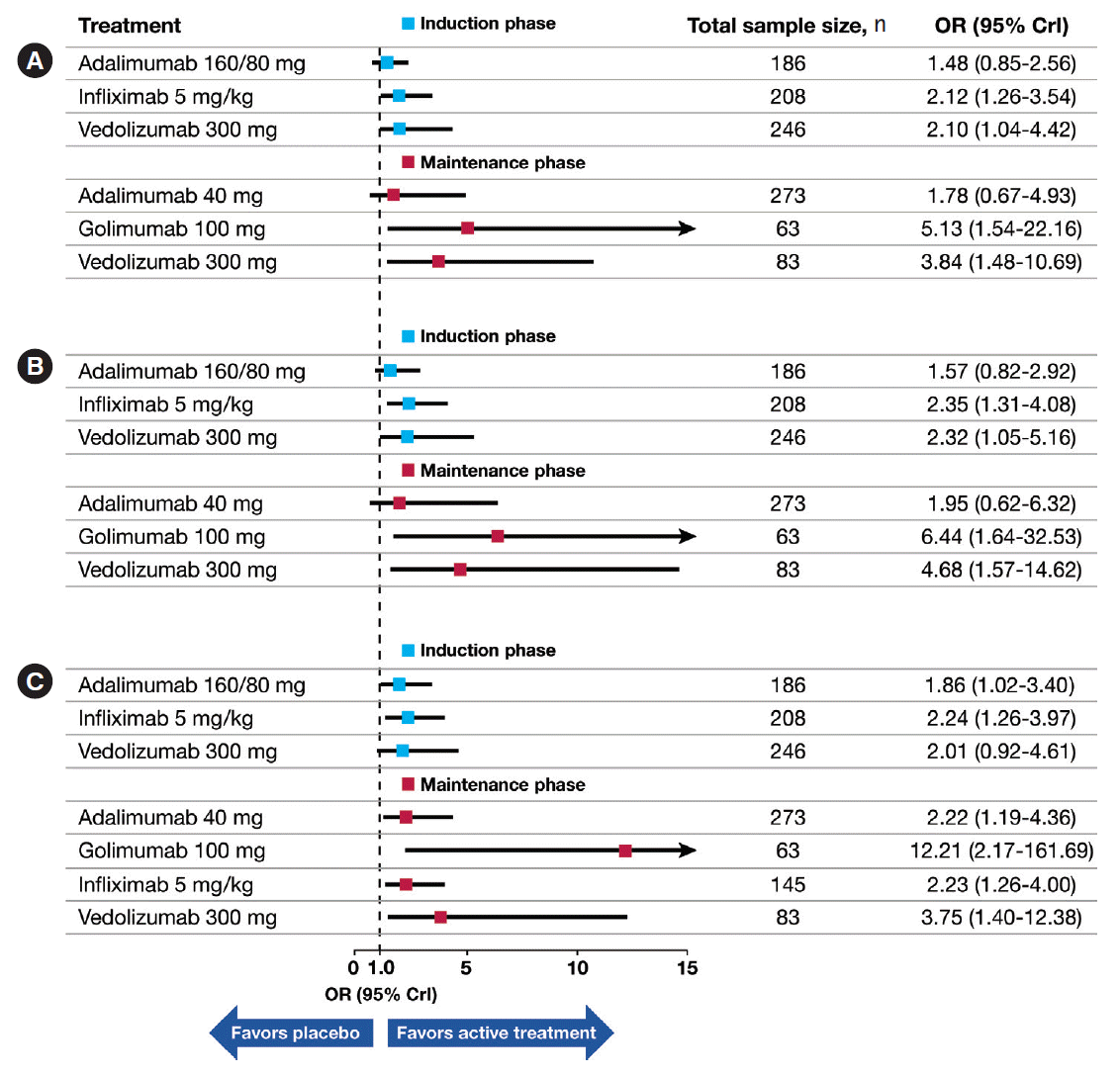
Table 1.
| Author (year) | Study identifier | Study phase | Treatment phase | Study treatments | Sample size, n | Male sex (%) | Mean age (yr) | Mean disease duration (yr) | Mean score |
|---|---|---|---|---|---|---|---|---|---|
| Hibi et al. (2017) [16] | NCT01863771 (PURSUIT-J) | 3 | Maintenance | Placebo | 31 | 61 | 42.9 | 5.7a | 8.0a |
| Golimumab (SC) 100 mg Q4W | 32 | 59 | 39.3 | 5.4a | 8.0a | ||||
| Kobayashi et al. (2016) [17] | Japic CTI-060298 | 3 | Induction | Placebo | 104 | 64 | 37.8 | 7.1 | 8.5 |
| Infliximab (IV) 5 mg/kg wk 0, 2, 6 | 104 | 63 | 40.0 | 8.1 | 8.6 | ||||
| Maintenance | Placebo | 72 | NA | NA | NA | NA | |||
| Infliximab (IV) 5 mg/kg wk 14, 22 | 73 | NA | NA | NA | NA | ||||
| Suzuki et al. (2014) [19] | NCT00853099 | 2/3 | Induction | Placebo | 96 | 73 | 41.3 | 7.8 | 8.5 |
| Adalimumab (SC) 160 mg wk 0, 80 mg wk 2 | 90 | 68 | 42.5 | 7.8 | 8.6 | ||||
| Maintenance | Placebo | 96 | 73 | 41.3 | 7.8 | 8.5 | |||
| Adalimumab (SC) 40 mg Q2W | 177 | 63 | 43.4 | 8.0 | 8.6 | ||||
| Motoya et al. (2019) [18] | NCT02039505 | 3 | Induction | Placebo | 82 | 67 | 44.0 | 8.6 | 8.1 |
| Vedolizumab (IV) 300 mg wk 0, 2, 6 | 164 | 60 | 42.3 | 7.2 | 8.3 | ||||
| Maintenance | Placebo | 42 | 55 | 42.6 | 8.7 | 7.9 | |||
| Vedolizumab (IV) 300 mg Q8W | 41 | 51 | 43.0 | 8.6 | 8.1 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download