Abstract
Background/Aims
Methods
Results
Conclusions
Notes
FINANCIAL SUPPORT
This study was sponsored by Pfizer. Medical writing support was provided by Sharmy Blows of Engage Scientific, Horsham, UK, and was funded by Pfizer.
CONFLICT OF INTEREST
This study was sponsored by Pfizer. Banerjee A, Isogawa N, Li Y, and Hassan-Zahraee M are employees of Pfizer. Ahmad A, Cataldi F, Gorelick KJ, Nagaoka M, and Clare R were employees of Pfizer during the OPERA study. Saruta M received lecture fees from Mitsubishi Tanabe Pharma, AbbVie GK, and Takeda Pharmaceutical Co., Ltd., and research grants from EA Pharma Co., Ltd., Kissei Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Mochida Pharmaceutical Co., Ltd. Watanabe M has the following disclosures: honoraria from Mitsubishi Tanabe Pharma Corp., Eisai Co., Ltd., Kyorin Pharmaceutical Co., Ltd., JIMRO Co., Ltd., AbbVie GK, Takeda Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Zeria Pharmaceutical Co., Ltd., Asahi Kasei Medical Co., Ltd., EA Pharma Co., Ltd., Astellas Pharma Inc., Mochida Pharmaceutical Co., Ltd., Janssen Pharmaceutical Co., Ltd., Gilead Sciences, Inc., Celgene Corp., KISSEI Pharmaceutical Co., Ltd., and commercial research funding from: Asahi Kasei Medical Co., Ltd., AbbVie GK, EA Pharma Co., Ltd., Eisai Co., Ltd., Kyorin Phar maceutical Co., Ltd., Mitsubishi Tanabe Pharma Corp., Otsuka Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Zeria Pharmaceutical Co., Ltd., JIMRO Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Mochida Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Astellas Pharma Inc., MSD K.K., Dainippon Sumitomo Dainippon Pharma Co., Ltd., Bristol-Myers, K.K, Chugai Pharmaceutical Co., Ltd., Gilead Sciences, Inc., Pfizer Inc., Miyarisan Pharmaceutical Co., Ltd., KISSEI Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd. Jang BI, Park DI, Cheon JH, Im JP, Yang SK, Hibi T, Kanai T, Katsuno T, Kim YH, and Li Y have no disclosures. However, all of these are not relevant to this article.
ACKNOWLEDGMENTS
REFERENCES
Fig. 1.
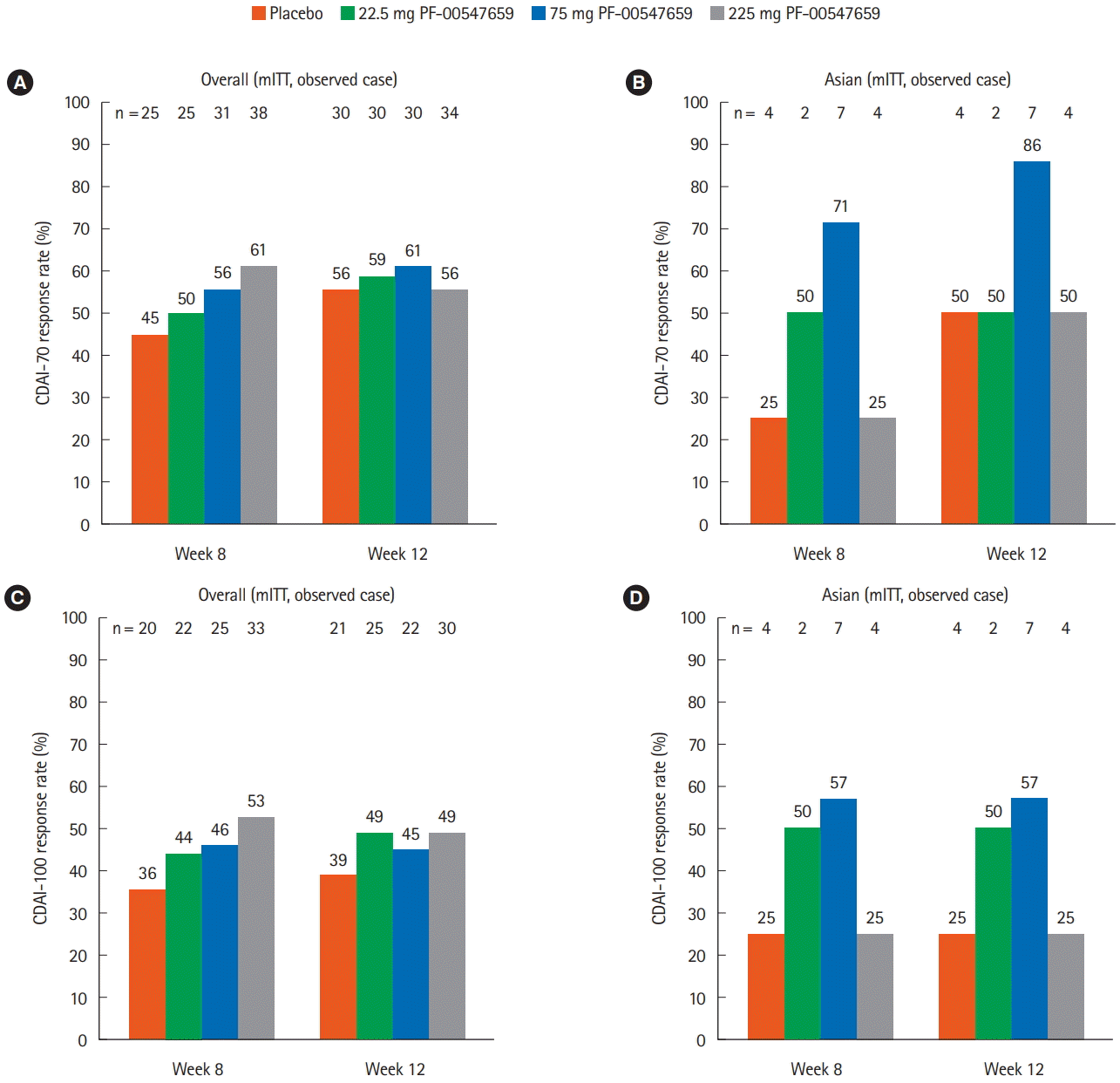
Fig. 2.
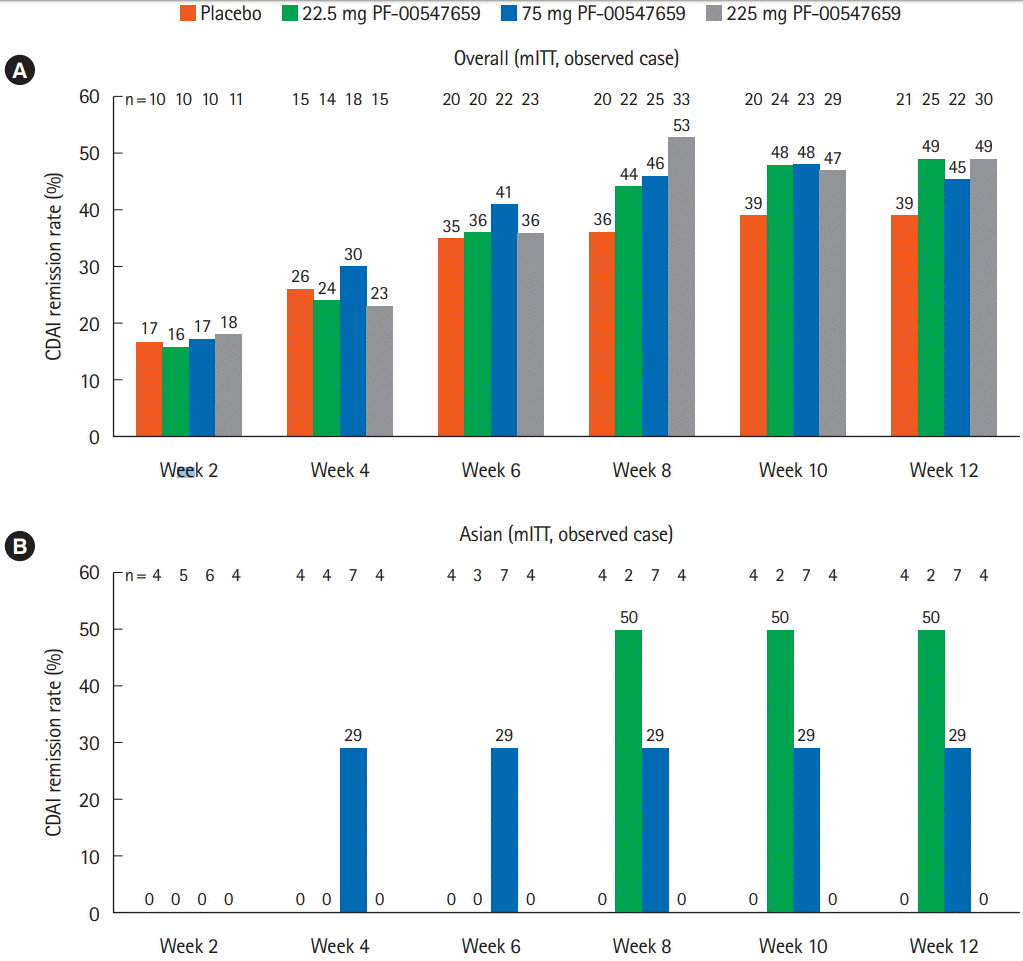
Fig. 3.
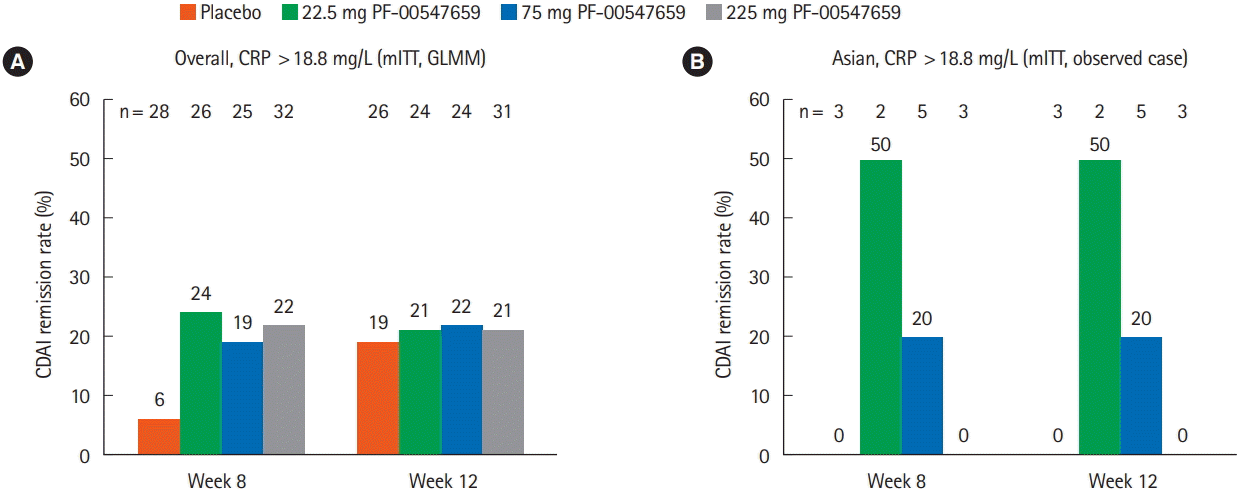
Fig. 4.
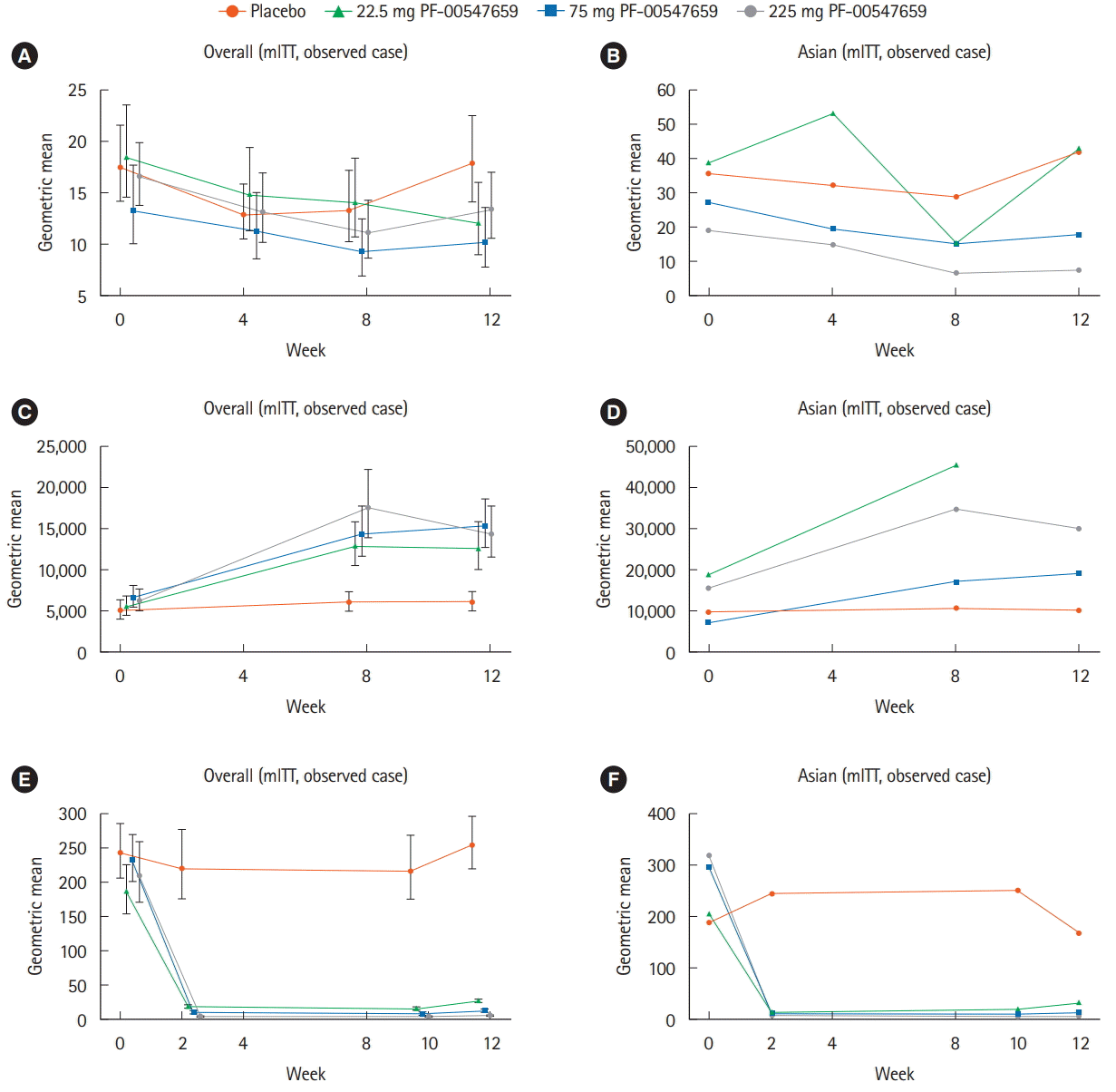
Table 1.
Table 2.
| PK parameter |
Treatment |
||||||
|---|---|---|---|---|---|---|---|
|
22.5 mg |
75 mg |
225 mg |
|||||
| Non-Japanese (n=7) | Japanese (n=3) | Non-Japanese (n=7) | Ratioa | Japanese (n=3) | Non-Japanese (n=10) | Ratioa | |
| AUC648 (μg · hr/mL) | 550 (53) | 3,399 (16) | 4,620 (32) | 0.74 | 15,630 (39) | 9,608 (41)b | 1.63 |
| Cmax (μg/mL) | 1.756 (45) | 9.454 (26) | 11.430 (19) | 0.83 | 35.360 (15) | 21.480 (47) | 1.65 |
| Tmax (hr) | 140 (44–333) | 144 (125–170) | 142 (45–171) | - | 165 (144–166) | 154 (52–214) | - |
| t½ (day) | 4.977 ± 2.844c | 7.907 ± 1.713 | 9.288 ± 2.713d | - | 7.870 ± 11.900e | 13.380 ± 4.040f | - |
Table 3.
| Population |
Placebo |
22.5 mg |
75 mg |
225 mg |
All active treatment |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Asian | Overall | Asian | Overall | Asian | Overall | Asian | Overall | Asian | |
| No. | 63 | 4 | 66 | 5 | 65 | 7 | 68 | 4 | 199 | 16 |
| Subjects with AEs | 54 (86) | 3 (75) | 57 (85) | 4 (80) | 51 (80) | 4 (57) | 53 (78) | 4 (100) | 161 (81) | 12 (75) |
| Subjects with serious AEs | 5 (8) | 0 | 11 (16) | 1 (20) | 9 (14) | 0 | 11 (16) | 0 | 31 (16) | 1 (6) |
| Discontinuations because of AE | 3 (5) | 0 | 9 (13) | 2 (40) | 8 (13) | 0 | 4 (6) | 0 | 21 (11) | 2 (13) |
| CD | 3 (5) | 0 | 4 (6) | 0 | 3 (5) | 0 | 2 (3) | 0 | 9 (5) | 0 |
| CD-related | 0 | 0 | 2 (3) | 1 (20) | 3 (5) | 0 | 1 (1) | 0 | 6 (3) | 1 (6) |
| Other | 0 | 0 | 3 (4) | 1 (20) | 2 (3) | 0 | 1 (1) | 0 | 6 (3) | 1 (6) |
| Infections | 20 (32) | 1 (25) | 27 (40) | 1 (20) | 25 (39) | 2 (29) | 24 (35) | 3 (75) | 76 (38) | 6 (38) |
| GI tract/liver infectionsa | 1 (2) | 0 | 8 (12) | 0 | 7 (11) | 1 (14) | 4 (6) | 0 | 19 (10) | 1 (6) |
| Infections in ex-GI MAdCAM bearing tissues | 10 (16) | 0 | 6 (9) | 0 | 6 (9) | 1 (14) | 8 (12) | 2 (50) | 20 (10) | 3 (19) |
| Nasopharyngitis | 5 (8) | 0 | 3 (4) | 0 | 4 (6) | 1 (14) | 5 (7) | 1 (25) | 12 (6) | 2 (13) |
| Bladder | 5 (8) | 0 | 3 (4) | 0 | 2 (3) | 0 | 3 (4) | 1 (25) | 8 (4) | 1 (6) |
| Other (spleen, uterus) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
a Anal abscess, anal fistula infection, appendicitis. Gastroenteritis, gastroenteritis viral, GI inflammation, GI infection, GI viral infection, liver abscess, perirectal abscess, and rectal abscess (MedDRA version 18.1). [10]




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download